August 27, 2024
2024 The Most Effective Bpc-157 Powder Distributor Pdf
Bpc-157 Frameworks of six metabolites determined by high-performance liquid chromatography-tandem mass spectrometry in rat plasma, bile, urine, and feces complying with a single intramuscular administration of 100 µg/ 300 μCi/ kg of [3H] BPC157. In the abovementioned researches, we characterized the pharmacokinetic profile of prototype BPC157 making use of high-performance fluid chromatography (HPLC) in rats and dogs. Next, we examined the excretion, metabolism, and cells distribution of BPC157 in rats after a single IM shot of 100 µg/ 300 μCi/ kg [3H] BPC157. [3H] BPC157 was well tolerated by all rats, and no aesthetic indicators of toxicity were observed. Prolines of BPC157 were labeled with [3H] and the framework of [3H] -classified BPC157 is shown in Figure 3A. The problems of the FDA pertaining to BPC 157 mostly entail safety and security considerations and the absence of extensive medical trials.Bpc-157
- In calvarial window (top), at 15 minutes enhanced stress time and medication saline (5 ml/kg ip) (upper, left, control, a) or BPC 157 (10 ng/kg sc) (top, ideal, A), at 10 min enhanced intra-abdominal pressure time.
- BPC 157 might be valuable for people who are trying to find an anti-inflammatory agent.
- In the initial cycle, a normal saline remedy (6 μg/ kg) of BPC157 was provided intravenously.
- In the 3rd cycle, the canines were provided 30 μg/ kg BPC157 saline solution by IM injection once daily for seven successive days.
Regularly Asked Concerns About Bpc-157
People coming to grips with gut-related distress observe enhancements, marking the peptide as a potential ally for a host of digestive problems. Envision ligaments weaving back to stamina, abscess yielding to restoration, and inflamed cells finding solace in the peptide's restorative embrace. This effective compound, when mostly connected to recovery easy lacerations, now depends on the cusp of redefining therapy techniques for a breadth of conditions, its potential surging bent on touch lives with recovery luck. As anticipated, the tail motor feature ratings shown consistent debilitation in the rats that underwent spine injury and received saline postinjury. As a result, BPC 157 treatment was administered by an one-time intraperitoneal injection (BPC 157 (200 or 2 μg/ kg) or 0.9% NaCl (5 ml/kg)) 10 min after injury. The injury procedure included laminectomy (degree L2-L3) and a 60-s compression (neurosurgical piston (60-- 66 g) of the revealed dural cavity of the sacrocaudal spine).Analyzing Research Study Outcomes For Various Types Of Administration
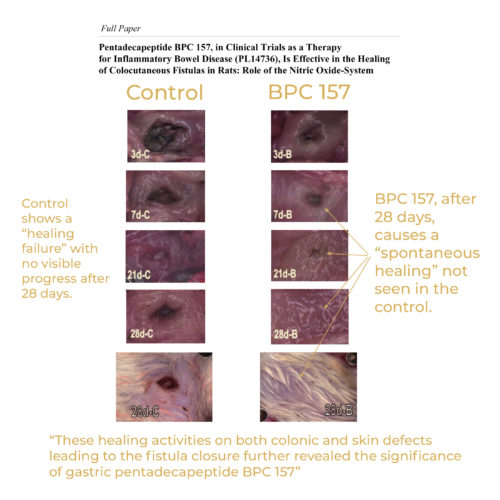
https://Clinical-trials.b-cdn.net/Clinical-trials/regenerative-medicine/what-is-bpc-157-potential-uses.html Nonetheless, the complete extent of advantages might take longer to show up, specifically for chronic or extreme conditions. Uniformity in use and adherence to recommended dosages are essential consider attaining optimal results. In this procedure, particular chemicals are integrated in a regulated environment to produce the peptide. Yet, there's one more peptide called Pentadecapeptide Arginate (Personal Organizer or PDA-Biopeptide), closely appearing like BPC-157. It coincides variation with the exact same 15 amino acid sequence as BPC-157, however with an included arginate salt for much better security. A cam affixed to a VMS-004 Exploration Deluxe USB microscope (Veho, United States) was used for recording. In deeply anesthetized rats, laparatomized prior to sacrifice, we examined the gross sores in the intestinal system and in the belly (amount of the lengthiest diameters, mm) (Gojkovic et al., 2020; Kolovrat et al., 2020; Gojkovic et al., 2021a; Knezevic et al., 2021a; Knezevic et al., 2021a; Gojkovic et al., 2021b; Knezevic et al., 2021b; Strbe et al., 2021). The typical healing rates of overall radioactivity in pee, feces, and cage cleaning fluid accumulated from 0 to 72 h after [3H] BPC157 management in intact rats were 15.88% ± 2.99%, 2.25% ± 0.67%, and 1.41% ± 1.04%, respectively, and the proportion of recurring radioactivity in the cadavers was 54.31% ± 3.04% (Table 7; Figure 3B). Similarly, starting on day 7, the controls displayed edema and the loss of nerve cells in the anterior horn and intermediate noodle, disruptions that were largely combated the in BPC 157-treated rats (Table 2 and Fig. 5). Prior to sacrifice, the pets from the 30-, 90-, 180-, and 360-day postspinal cable injury interval groups were put in a wood box with their tails revealed. Three pairs of monopolar needles were stabbed 3 mm deep right into the tail 10, 60, and 100 mm caudal to the tail base. Utilizing a TECA 15 electromyography apparatus with a signal filter between 50 Hz and 5 kHz, volunteer muscle task was tape-recorded from the most back set of electrodes, and the ordinary motor device potential (MUP) was recorded. Thereafter, the compound electric motor action potential (CMAP) was tape-recorded from the very same set of electrodes after stimulating the initial and 2nd electrodes (a repetition of 1 Hz and a stimulation period of 0.05 ms). Control rats showed within cerebellar location karyopyknosis and deterioration of Purkinje cells (a, b). Significant and dynamic karyopyknosis and deterioration of pyramidal cell of the hippocampus was observed in control rats (arrowheads) at 25 mmHg intraabdominal stress (c) and much more at 50 mmHg intra-abdominal stress (d). No change was discovered in the cerebellar and hippocampal location in BPC 157- dealt with rats at 25 mmHg intra-abdominal stress (A, B, C) and only uncommon hippocampal karyopyknotic cells (arrowheads) at 50 mmHg intra-abdominal pressure (D) (HE; magnifying × 400, scale bar 50 μm). Similarly, in the cause-consequence course of the therapy, BPC 157 decreased apoplexy, both peripherally and centrally. Without treatment, apoplexy imminently occurred in addition to high intra-abdominal pressure, peripherally in veins (i.e., portal blood vessel and substandard caval vein, remarkable mesenteric vein, hepatic veins, and outside throaty vein) and in arteries (i.e., exceptional mesenteric artery, hepatic artery and stomach aorta) and centrally (i.e., premium sagittal sinus) (Number 6). As a synthetic peptide, BPC 157's standing requires cautious evaluation by regulatory bodies like the FDA. Discover the reality behind the 'BPC 157 outlawed' headings in our most current exploration. The FDA's choice pertaining to BPC 157, a peptide understood for its possible recovery homes, has caused a stir in the health and wellness neighborhood. Widely discussed because of its popularity, this growth has actually opened up a range of point of views and discussions. In this article, we dive into the diverse perspectives on BPC 157's advantages and the FDA's decision. In different team of animals, death was evaluated daily until post-operative day 7, as defined formerly [13,18]The Tragic Connection Between Ehlers-Danlos and Arachnoiditis - Pain News Network
The Tragic Connection Between Ehlers-Danlos and Arachnoiditis.
Posted: Thu, 18 May 2023 07:00:00 GMT [source]
The length of time has BPC 157 been around?
The BPC-157 peptide''s background starts with the discovery of the compound by a Croatian scientific group in the very early 1990s. Since then, the therapeutic possibility of the BPC-157 peptide has been extensively explored.

Social Links