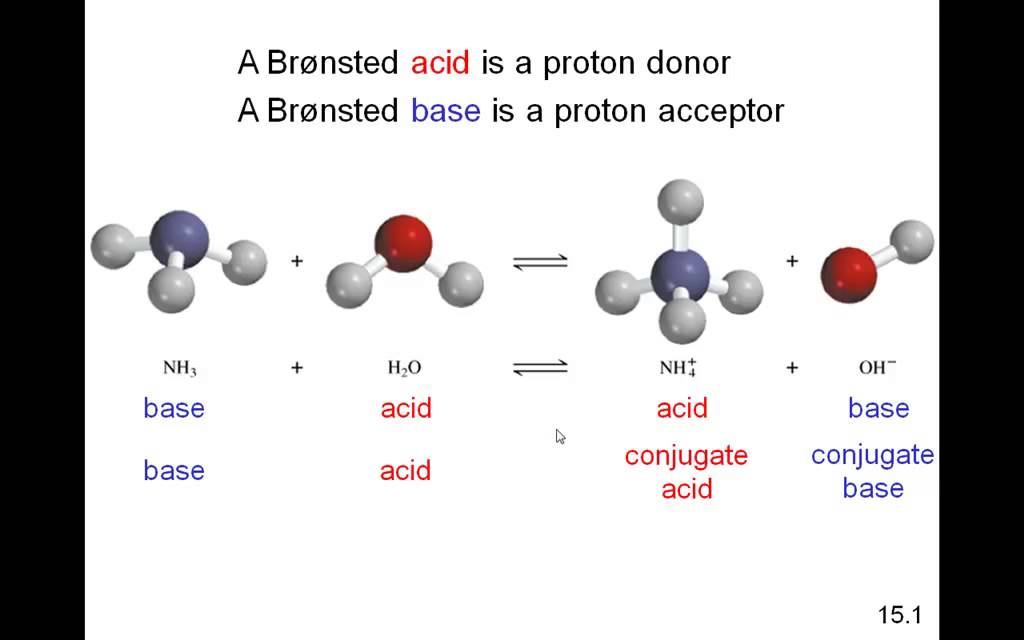
What Is A Property Of Base . Learn about the properties of bases and see examples of bases and their uses. Bases are substances that taste bitter and feel slippery when dissolved in water. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. They are commonly found in household items like baking soda and soap. A base is a substance to which a proton (h +) can be added. Aqueous solutions of bases are also electrolytes. Essentially, an acid donates protons to bases. Bases have properties that mostly contrast with those of acids.
from www.youtube.com
Bases have properties that mostly contrast with those of acids. A base is a substance to which a proton (h +) can be added. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Bases are substances that taste bitter and feel slippery when dissolved in water. They are commonly found in household items like baking soda and soap. Aqueous solutions of bases are also electrolytes. Learn about the properties of bases and see examples of bases and their uses. Essentially, an acid donates protons to bases.
Bronsted Acids & Bases & Acid Base Properties of Water (Crisanti) YouTube
What Is A Property Of Base Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. A base is a substance to which a proton (h +) can be added. Learn about the properties of bases and see examples of bases and their uses. Bases have properties that mostly contrast with those of acids. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Aqueous solutions of bases are also electrolytes. Bases are substances that taste bitter and feel slippery when dissolved in water. They are commonly found in household items like baking soda and soap. Essentially, an acid donates protons to bases.
From www.geeksforgeeks.org
ACID Properties in DBMS What Is A Property Of Base Bases are substances that taste bitter and feel slippery when dissolved in water. Essentially, an acid donates protons to bases. They are commonly found in household items like baking soda and soap. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts. What Is A Property Of Base.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Is A Property Of Base Bases have properties that mostly contrast with those of acids. Bases are substances that taste bitter and feel slippery when dissolved in water. They are commonly found in household items like baking soda and soap. Learn about the properties of bases and see examples of bases and their uses. A base is a substance to which a proton (h +). What Is A Property Of Base.
From www.youtube.com
BASE properties in DBMS (with example) YouTube What Is A Property Of Base Aqueous solutions of bases are also electrolytes. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. They are commonly found in household items like baking soda and soap. Essentially, an acid donates protons to bases. A base is a. What Is A Property Of Base.
From www.cuemath.com
Change of Base Formula What Is Change of Base Formula? Formula What Is A Property Of Base Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. A base is a substance to which a proton (h +) can be added. Base,. What Is A Property Of Base.
From www.showme.com
4.3 Properties of Logarithms, Change of Base Math ShowMe What Is A Property Of Base Aqueous solutions of bases are also electrolytes. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. They are commonly found in household items like baking soda and soap. Base, in chemistry, any substance that in water solution is slippery to the. What Is A Property Of Base.
From www.coursehero.com
[Solved] D sold capital property in the current year for net proceeds What Is A Property Of Base They are commonly found in household items like baking soda and soap. Bases are substances that taste bitter and feel slippery when dissolved in water. A base is a substance to which a proton (h +) can be added. Essentially, an acid donates protons to bases. Learn about the properties of bases and see examples of bases and their uses.. What Is A Property Of Base.
From www.ck12.org
Exponential Properties Involving Products CK12 Foundation What Is A Property Of Base They are commonly found in household items like baking soda and soap. Aqueous solutions of bases are also electrolytes. Essentially, an acid donates protons to bases. A base is a substance to which a proton (h +) can be added. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions,. What Is A Property Of Base.
From www.tpsearchtool.com
How To Change Base Of Log Images What Is A Property Of Base Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Bases are substances that taste bitter and feel slippery when dissolved in water. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids.. What Is A Property Of Base.
From studylib.net
Properties of acids and bases What Is A Property Of Base Learn about the properties of bases and see examples of bases and their uses. They are commonly found in household items like baking soda and soap. Bases are substances that taste bitter and feel slippery when dissolved in water. Aqueous solutions of bases are also electrolytes. Base, in chemistry, any substance that in water solution is slippery to the touch,. What Is A Property Of Base.
From www.slideserve.com
PPT Solutions, Acids, and Bases PowerPoint Presentation, free What Is A Property Of Base Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Bases are substances that taste bitter and feel slippery when dissolved in water. They are commonly found in household items like baking soda and soap. In chemistry, a base is a substance that reacts with acids to form a salt and which releases. What Is A Property Of Base.
From www.youtube.com
Bronsted Acids & Bases & Acid Base Properties of Water (Crisanti) YouTube What Is A Property Of Base Essentially, an acid donates protons to bases. They are commonly found in household items like baking soda and soap. Learn about the properties of bases and see examples of bases and their uses. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue),. What Is A Property Of Base.
From www.youtube.com
Change of Base Formula Logarithms YouTube What Is A Property Of Base Bases are substances that taste bitter and feel slippery when dissolved in water. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Bases have properties that mostly contrast with those of acids. Base, in chemistry, any substance that in water solution. What Is A Property Of Base.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation, free What Is A Property Of Base Bases have properties that mostly contrast with those of acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Bases are substances that taste bitter and feel slippery when dissolved in water. Learn about the properties of bases and see examples. What Is A Property Of Base.
From www.youtube.com
Base changing properties of Logarithms (Part 3) Lecture 7 logab What Is A Property Of Base Bases are substances that taste bitter and feel slippery when dissolved in water. A base is a substance to which a proton (h +) can be added. They are commonly found in household items like baking soda and soap. Aqueous solutions of bases are also electrolytes. Essentially, an acid donates protons to bases. Base, in chemistry, any substance that in. What Is A Property Of Base.
From www.expii.com
Conjugate AcidBase Pairs — Overview & Examples Expii What Is A Property Of Base Aqueous solutions of bases are also electrolytes. A base is a substance to which a proton (h +) can be added. Bases have properties that mostly contrast with those of acids. Bases are substances that taste bitter and feel slippery when dissolved in water. Learn about the properties of bases and see examples of bases and their uses. Base, in. What Is A Property Of Base.
From www.sliderbase.com
AcidBase Reactions Presentation Chemistry What Is A Property Of Base In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. A base is a substance to which a proton (h +) can be added. Essentially, an acid donates protons to bases. Learn about the properties of bases and see examples of bases. What Is A Property Of Base.
From www.slideserve.com
PPT Properties of Logarithms PowerPoint Presentation, free download What Is A Property Of Base In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Essentially, an acid donates protons to bases. Aqueous solutions of bases are also electrolytes. Bases are substances that taste bitter and feel slippery when dissolved in water. They are commonly found in. What Is A Property Of Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6523668 What Is A Property Of Base Learn about the properties of bases and see examples of bases and their uses. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Essentially, an acid donates protons to bases. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons,. What Is A Property Of Base.
From www.propertybase.com
Real Estate Software CRM, Back Office, Lead Generation, Marketing & More What Is A Property Of Base Bases are substances that taste bitter and feel slippery when dissolved in water. Essentially, an acid donates protons to bases. A base is a substance to which a proton (h +) can be added. Aqueous solutions of bases are also electrolytes. They are commonly found in household items like baking soda and soap. Bases have properties that mostly contrast with. What Is A Property Of Base.
From www.slideserve.com
PPT Common Bases PowerPoint Presentation, free download ID2782494 What Is A Property Of Base Bases are substances that taste bitter and feel slippery when dissolved in water. They are commonly found in household items like baking soda and soap. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. In chemistry, a base is a substance that reacts with acids to form a salt and which releases. What Is A Property Of Base.
From www.slideserve.com
PPT Properties of Logarithms PowerPoint Presentation, free download What Is A Property Of Base Bases are substances that taste bitter and feel slippery when dissolved in water. A base is a substance to which a proton (h +) can be added. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. They are commonly found in. What Is A Property Of Base.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Is A Property Of Base Aqueous solutions of bases are also electrolytes. A base is a substance to which a proton (h +) can be added. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Learn about the properties of bases and see examples of bases. What Is A Property Of Base.
From brilliant.org
Properties of Isosceles Triangles Brilliant Math & Science Wiki What Is A Property Of Base Essentially, an acid donates protons to bases. They are commonly found in household items like baking soda and soap. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. Learn about the properties of bases and see examples of bases. What Is A Property Of Base.
From www.slideserve.com
PPT Acid and Bases An Introduction PowerPoint Presentation, free What Is A Property Of Base Learn about the properties of bases and see examples of bases and their uses. Essentially, an acid donates protons to bases. Bases have properties that mostly contrast with those of acids. They are commonly found in household items like baking soda and soap. In chemistry, a base is a substance that reacts with acids to form a salt and which. What Is A Property Of Base.
From www.cuemath.com
Properties of Log What are Logarithmic Properties? What Is A Property Of Base Aqueous solutions of bases are also electrolytes. Learn about the properties of bases and see examples of bases and their uses. Bases have properties that mostly contrast with those of acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Base,. What Is A Property Of Base.
From studylib.net
ACID BASE CHEMISTRY What Is A Property Of Base In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Aqueous solutions of bases are also electrolytes. They are commonly found in household items like baking soda and soap. Learn about the properties of bases and see examples of bases and their. What Is A Property Of Base.
From www.teachoo.com
Uses of Base 5+ Examples Chemistry Teachoo Teachoo Questions What Is A Property Of Base Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. They are commonly found in household items like baking soda and soap. Learn about the properties of bases and see examples of bases and their uses. Aqueous solutions of bases. What Is A Property Of Base.
From sciencenotes.org
Associative Property in Math Definition and Examples What Is A Property Of Base A base is a substance to which a proton (h +) can be added. Bases are substances that taste bitter and feel slippery when dissolved in water. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Bases have properties that mostly. What Is A Property Of Base.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free What Is A Property Of Base Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. A base is a substance to which a proton (h +) can be added. Essentially, an acid donates protons to bases. They are commonly found in household items like baking. What Is A Property Of Base.
From www.slideserve.com
PPT A Very Brief Introduction to Relational Databases PowerPoint What Is A Property Of Base Essentially, an acid donates protons to bases. A base is a substance to which a proton (h +) can be added. Aqueous solutions of bases are also electrolytes. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. They are commonly found. What Is A Property Of Base.
From www.worksheetsplanet.com
What is a Base Definition of Base What Is A Property Of Base They are commonly found in household items like baking soda and soap. Aqueous solutions of bases are also electrolytes. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. Learn about the properties of bases and see examples of bases. What Is A Property Of Base.
From classfullkatmandu.z19.web.core.windows.net
What Is A Negative Exponent Rule What Is A Property Of Base A base is a substance to which a proton (h +) can be added. Essentially, an acid donates protons to bases. They are commonly found in household items like baking soda and soap. Learn about the properties of bases and see examples of bases and their uses. Aqueous solutions of bases are also electrolytes. Base, in chemistry, any substance that. What Is A Property Of Base.
From www.slideserve.com
PPT Logarithm Properties Change of Base PowerPoint Presentation What Is A Property Of Base Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Bases are. What Is A Property Of Base.
From www.youtube.com
Logarithms Change of Base Property YouTube What Is A Property Of Base Bases have properties that mostly contrast with those of acids. Essentially, an acid donates protons to bases. Aqueous solutions of bases are also electrolytes. They are commonly found in household items like baking soda and soap. Bases are substances that taste bitter and feel slippery when dissolved in water. Base, in chemistry, any substance that in water solution is slippery. What Is A Property Of Base.
From mathsathome.com
How to Change the Base of a Logarithm What Is A Property Of Base They are commonly found in household items like baking soda and soap. Aqueous solutions of bases are also electrolytes. Bases are substances that taste bitter and feel slippery when dissolved in water. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution.. What Is A Property Of Base.