Strength Of H Bond Is Maximum In . In this section, you will learn. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. As has already been brought up, this question is not easy to answer. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are.
from www.slideserve.com
Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. In this section, you will learn. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. As has already been brought up, this question is not easy to answer. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures.
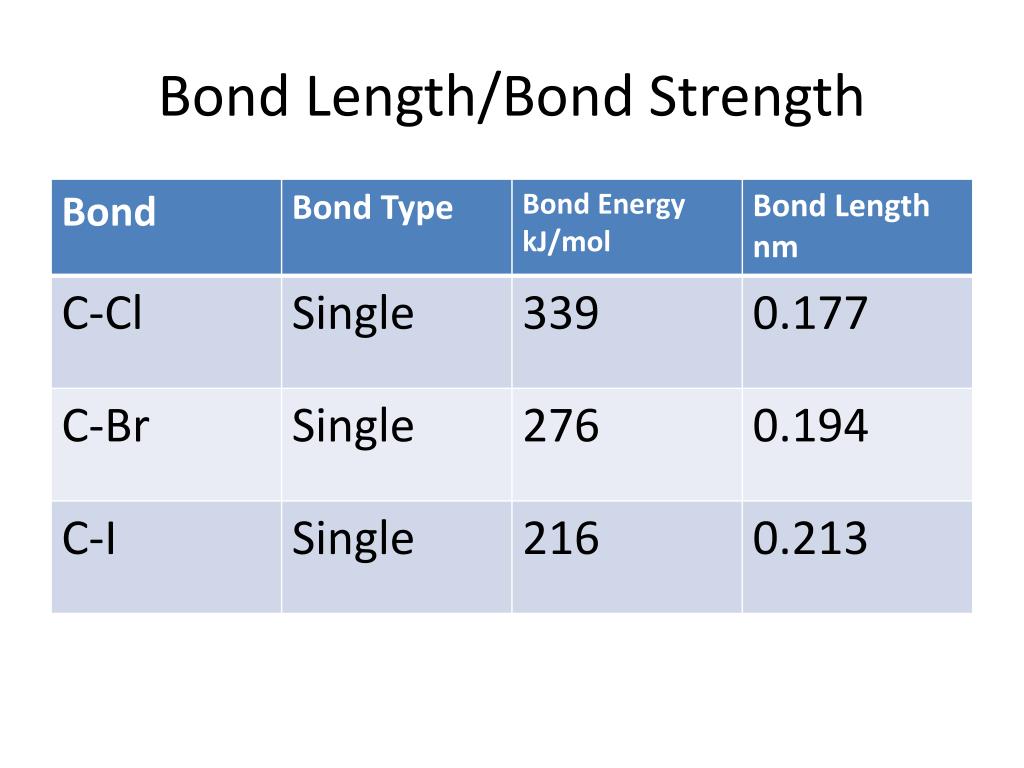
PPT Bond Strength, Bond Length, and Bond Energy PowerPoint
Strength Of H Bond Is Maximum In Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. As has already been brought up, this question is not easy to answer. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. In this section, you will learn. The quick answer is that ice has stronger hydrogen bonds than liquid water on average.
From www.slideserve.com
PPT The name is bondH bond Akhil Khanal February 2006 PowerPoint Strength Of H Bond Is Maximum In The quick answer is that ice has stronger hydrogen bonds than liquid water on average. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. Bonds between hydrogen and atoms in the same column of. Strength Of H Bond Is Maximum In.
From www.masterorganicchemistry.com
Bond Dissociation Energies = Homolytic Cleavage — Master Organic Chemistry Strength Of H Bond Is Maximum In A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. The quick answer is that ice has stronger hydrogen bonds than liquid water. Strength Of H Bond Is Maximum In.
From www.numerade.com
Answer the following questions about bond length and strength. Part 1 Strength Of H Bond Is Maximum In As has already been brought up, this question is not easy to answer. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. The quick answer is. Strength Of H Bond Is Maximum In.
From www.researchgate.net
Relationship between MH bond strength and work function, for dmetals Strength Of H Bond Is Maximum In The quick answer is that ice has stronger hydrogen bonds than liquid water on average. In this section, you will learn. As has already been brought up, this question is not easy to answer. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. A bond’s strength describes. Strength Of H Bond Is Maximum In.
From www.researchgate.net
Bondstrengthbondlength parameters for MO bonds (corrected for Strength Of H Bond Is Maximum In A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. As has already been brought up, this question is not. Strength Of H Bond Is Maximum In.
From www.chegg.com
Solved Bond TABLE 10.3 Average Bond Energies Bond Energy Strength Of H Bond Is Maximum In We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. In this section, you. Strength Of H Bond Is Maximum In.
From www.masterorganicchemistry.com
Bond Strengths And Radical Stability Master Organic Chemistry Strength Of H Bond Is Maximum In We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. A bond’s strength describes. Strength Of H Bond Is Maximum In.
From www.chegg.com
Solved Use The Bond Dissociation Energies From The Table Strength Of H Bond Is Maximum In In this section, you will learn. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. A. Strength Of H Bond Is Maximum In.
From www3.nd.edu
Hydrogen Bond Lengths in Biomolecules Strength Of H Bond Is Maximum In Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out. Strength Of H Bond Is Maximum In.
From www.chegg.com
Consult Table 3.2 to find the singlebond energies Strength Of H Bond Is Maximum In Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. A bond’s strength describes. Strength Of H Bond Is Maximum In.
From www.coursehero.com
[Solved] rank the bond dissociation energy of the indicated CH bonds Strength Of H Bond Is Maximum In Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. We can determine the maximum number of. Strength Of H Bond Is Maximum In.
From www.researchgate.net
(a) Relationship between measured j 0 and the strength of the M−H bond Strength Of H Bond Is Maximum In The quick answer is that ice has stronger hydrogen bonds than liquid water on average. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. In this section, you will learn. As. Strength Of H Bond Is Maximum In.
From www.pathwaystochemistry.com
BondLengths Pathways to Chemistry Strength Of H Bond Is Maximum In Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. A bond’s strength describes how strongly each atom. Strength Of H Bond Is Maximum In.
From courses.lumenlearning.com
Strengths of Ionic and Covalent Bonds Chemistry Strength Of H Bond Is Maximum In A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. In this section, you will learn. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. Bonds between hydrogen and atoms in the same column of. Strength Of H Bond Is Maximum In.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID3842843 Strength Of H Bond Is Maximum In We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. The quick answer is that ice has stronger hydrogen bonds than liquid water. Strength Of H Bond Is Maximum In.
From pathwaystochemistry.com
Bond Length and Bond Strength Pathways to Chemistry Strength Of H Bond Is Maximum In Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out. Strength Of H Bond Is Maximum In.
From www.facebook.com
Celebrating our commitment to... Brother's Bond Bourbon Strength Of H Bond Is Maximum In Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. As has already been brought up, this question is not easy to answer. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. Bonds between hydrogen and atoms in the same column of the periodic. Strength Of H Bond Is Maximum In.
From www.slideserve.com
PPT Bond Strength, Bond Length, and Bond Energy PowerPoint Strength Of H Bond Is Maximum In Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. As has already been brought up, this question is not easy to answer. In this section, you will learn. A bond’s strength describes how strongly each atom is joined to. Strength Of H Bond Is Maximum In.
From schematiccntrylivinjhfxe.z21.web.core.windows.net
Energy Diagram Covalent Bond Strength Of H Bond Is Maximum In Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. As has already been brought up, this. Strength Of H Bond Is Maximum In.
From www.youtube.com
BOND LENGTH COMPARISON OF CC & CH bonds YouTube Strength Of H Bond Is Maximum In As has already been brought up, this question is not easy to answer. In this section, you will learn. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. The quick answer. Strength Of H Bond Is Maximum In.
From www.breakingatom.com
Bond Strength Strength Of H Bond Is Maximum In A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. In this. Strength Of H Bond Is Maximum In.
From pubs.acs.org
HydrogenBond Donors in Drug Design Journal of Medicinal Chemistry Strength Of H Bond Is Maximum In The quick answer is that ice has stronger hydrogen bonds than liquid water on average. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Bonds between. Strength Of H Bond Is Maximum In.
From quizlet.com
The bond lengths of carboncarbon, carbonnitrogen, carbono Quizlet Strength Of H Bond Is Maximum In A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. As has. Strength Of H Bond Is Maximum In.
From pt.slideshare.net
Tang 06 bond energy Strength Of H Bond Is Maximum In Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. As has already been brought up, this question is not easy to answer. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. We can determine the maximum number of hydrogen bonds formed. Strength Of H Bond Is Maximum In.
From www.coursehero.com
[Solved] Using the appropriate bond energies, calculate the heat of Strength Of H Bond Is Maximum In The quick answer is that ice has stronger hydrogen bonds than liquid water on average. In this section, you will learn. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. As has already been. Strength Of H Bond Is Maximum In.
From www.slideserve.com
PPT 1. Structure and Bonding PowerPoint Presentation, free download Strength Of H Bond Is Maximum In As has already been brought up, this question is not easy to answer. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Bonds between hydrogen and. Strength Of H Bond Is Maximum In.
From www.researchgate.net
Probabilities of Hbond formation between meloxicam and... Download Strength Of H Bond Is Maximum In We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. In this section, you will learn. As has already been brought up, this question is not easy to answer. A bond’s strength describes how strongly. Strength Of H Bond Is Maximum In.
From chemistry.stackexchange.com
Strength of Hydrogen Bond and Angle Chemistry Stack Exchange Strength Of H Bond Is Maximum In In this section, you will learn. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down. Strength Of H Bond Is Maximum In.
From www.slideserve.com
PPT Chemistry XL14A Chemical bonds PowerPoint Presentation, free Strength Of H Bond Is Maximum In Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. In this section, you will learn. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. We. Strength Of H Bond Is Maximum In.
From sciencenotes.org
Bond Energy and Strength Strength Of H Bond Is Maximum In Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. In this section, you will learn. As has already been. Strength Of H Bond Is Maximum In.
From www.chemistrysteps.com
Bond Length and Bond Strength Chemistry Steps Strength Of H Bond Is Maximum In As has already been brought up, this question is not easy to answer. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out. Strength Of H Bond Is Maximum In.
From www.chemistrysteps.com
Bond Length and Bond Strength Chemistry Steps Strength Of H Bond Is Maximum In We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out their lewis structures. Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is. Strength Of H Bond Is Maximum In.
From www.slideserve.com
PPT Chemistry XL14A Chemical bonds PowerPoint Presentation, free Strength Of H Bond Is Maximum In Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. The quick answer is that ice has stronger hydrogen bonds than liquid water on average. Hydrogen bond strengths range from 4 kj. Strength Of H Bond Is Maximum In.
From www.chemistrysteps.com
Bond Length and Bond Strength Chemistry Steps Strength Of H Bond Is Maximum In The quick answer is that ice has stronger hydrogen bonds than liquid water on average. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. As. Strength Of H Bond Is Maximum In.
From www.youtube.com
CHEMISTRY 101 Bond energies and bond lengths YouTube Strength Of H Bond Is Maximum In Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. We can determine the maximum number of hydrogen bonds formed for each molecule by drawing out. Strength Of H Bond Is Maximum In.