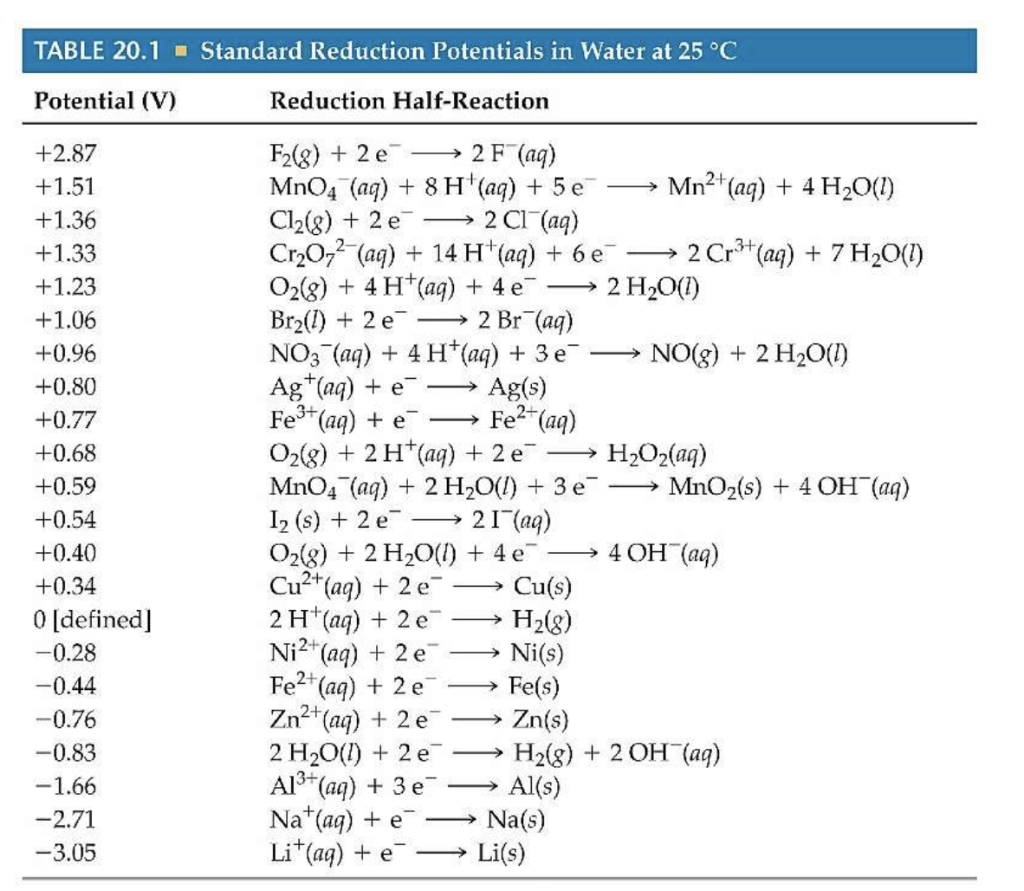
Standard Cell Potential Of Al . Only the difference between the potentials of two electrodes can be measured. Decode electrochemistry concepts with ease and. Interpret electrode potentials in terms of relative oxidant and reductant. Discover the spontaneity of redox reactions using our standard cell potential calculator. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods:
from mavink.com
Only the difference between the potentials of two electrodes can be measured. Interpret electrode potentials in terms of relative oxidant and reductant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Discover the spontaneity of redox reactions using our standard cell potential calculator. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. Decode electrochemistry concepts with ease and.
Tabel Potensial Standar
Standard Cell Potential Of Al The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Discover the spontaneity of redox reactions using our standard cell potential calculator. Only the difference between the potentials of two electrodes can be measured. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Decode electrochemistry concepts with ease and.
From www.numerade.com
SOLVED Cell Potentials The standard potential of a voltaic cell can be Standard Cell Potential Of Al The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Only the difference between the potentials of two electrodes. Standard Cell Potential Of Al.
From mungfali.com
Standard Cell Potential Equation Standard Cell Potential Of Al The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The standard cell potential (e°cell) is measured at 298. Standard Cell Potential Of Al.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Of Al The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Describe and relate the definitions of. Standard Cell Potential Of Al.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Of Al Describe and relate the definitions of electrode and cell potentials. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Discover the spontaneity of redox reactions using our standard cell potential calculator. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k. Standard Cell Potential Of Al.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Of Al Describe and relate the definitions of electrode and cell potentials. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Interpret electrode potentials in terms of relative oxidant and reductant. Only the difference between the potentials of two electrodes can be measured. Decode electrochemistry concepts with ease and.. Standard Cell Potential Of Al.
From www.youtube.com
Standard Cell Potentials YouTube Standard Cell Potential Of Al The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Discover the spontaneity of redox reactions using our standard cell potential calculator. Interpret electrode potentials in terms of relative oxidant and reductant. Only the difference between the potentials of two electrodes can be measured. The standard cell potential (\(e^o_{cell}\)) is the difference of. Standard Cell Potential Of Al.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Of Al Discover the spontaneity of redox reactions using our standard cell potential calculator. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. Decode electrochemistry concepts with ease and. The. Standard Cell Potential Of Al.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID165903 Standard Cell Potential Of Al Describe and relate the definitions of electrode and cell potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Discover the spontaneity of redox reactions using our standard cell potential calculator. Only. Standard Cell Potential Of Al.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Of Al Interpret electrode potentials in terms of relative oxidant and reductant. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. Standard Cell Potential Of Al.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Cell Potential Of Al The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant. Discover the spontaneity of redox reactions using our standard cell potential calculator. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode. Standard Cell Potential Of Al.
From www.chegg.com
Solved Calculate the standard cell potential at 25 degree C Standard Cell Potential Of Al Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Interpret electrode potentials in terms of relative oxidant and reductant. Only the difference between the potentials of two electrodes can be measured. Discover the spontaneity of redox reactions using our standard cell potential calculator. The potential. Standard Cell Potential Of Al.
From www.chegg.com
Solved Using the Standard Cell Potential Equation, Ecell = Standard Cell Potential Of Al Only the difference between the potentials of two electrodes can be measured. Discover the spontaneity of redox reactions using our standard cell potential calculator. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e°. Standard Cell Potential Of Al.
From www.slideserve.com
PPT Chemistry 30 Chapter 14 PowerPoint Presentation, free download Standard Cell Potential Of Al Discover the spontaneity of redox reactions using our standard cell potential calculator. Describe and relate the definitions of electrode and cell potentials. Decode electrochemistry concepts with ease and. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. This is also known. Standard Cell Potential Of Al.
From www.chegg.com
The standard potential for the following galvanic Standard Cell Potential Of Al The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Decode electrochemistry concepts with ease and. Discover the spontaneity of redox reactions using our standard cell potential calculator. Describe and relate the definitions of electrode and cell potentials. Only. Standard Cell Potential Of Al.
From pveducation.org
Standard Potential PVEducation Standard Cell Potential Of Al This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Discover. Standard Cell Potential Of Al.
From www.chegg.com
Solved Calculate the standard cell potential of the Standard Cell Potential Of Al The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. Decode electrochemistry concepts with ease and. Only the difference between the potentials of two electrodes can be measured. Interpret. Standard Cell Potential Of Al.
From www.slideserve.com
PPT Chemistry 30 Chapter 14 PowerPoint Presentation, free download Standard Cell Potential Of Al The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Only the difference between the potentials of two electrodes can be measured. Discover the spontaneity of redox reactions using our standard cell potential calculator. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods:. Standard Cell Potential Of Al.
From www.toppr.com
What will be standard cell potential of galvanic cell with the Standard Cell Potential Of Al Decode electrochemistry concepts with ease and. Discover the spontaneity of redox reactions using our standard cell potential calculator. Only the difference between the potentials of two electrodes can be measured. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential of the cell under standard conditions. Standard Cell Potential Of Al.
From www.numerade.com
SOLVED standard cell potentials Ecell = E cathode E anode Al3 Standard Cell Potential Of Al Interpret electrode potentials in terms of relative oxidant and reductant. Only the difference between the potentials of two electrodes can be measured. Discover the spontaneity of redox reactions using our standard cell potential calculator. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Describe and relate the. Standard Cell Potential Of Al.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID7041672 Standard Cell Potential Of Al Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The standard cell potential (e°cell) is measured at 298 k and all components in. Standard Cell Potential Of Al.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential Of Al The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Only the difference between the potentials of two electrodes can be measured. Discover the spontaneity of redox reactions using our standard cell potential. Standard Cell Potential Of Al.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Of Al Discover the spontaneity of redox reactions using our standard cell potential calculator. Decode electrochemistry concepts with ease and. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids. Standard Cell Potential Of Al.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Of Al Only the difference between the potentials of two electrodes can be measured. Interpret electrode potentials in terms of relative oxidant and reductant. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m. Standard Cell Potential Of Al.
From www.youtube.com
Electrochemistry 05 Calculating Standard Cell Potentials YouTube Standard Cell Potential Of Al The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Decode electrochemistry concepts with ease and. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe. Standard Cell Potential Of Al.
From mavink.com
Tabel Potensial Standar Standard Cell Potential Of Al 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Discover the spontaneity of redox reactions using our standard cell potential calculator. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode. Standard Cell Potential Of Al.
From www.bartleby.com
Answered 31)Calculate the cell potential for the… bartleby Standard Cell Potential Of Al 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Only the difference between the potentials of two electrodes. Standard Cell Potential Of Al.
From www.slideserve.com
PPT Lecture 2. Basic Electrochemistry PowerPoint Presentation, free Standard Cell Potential Of Al Decode electrochemistry concepts with ease and. Discover the spontaneity of redox reactions using our standard cell potential calculator. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The standard cell potential (e°cell) is. Standard Cell Potential Of Al.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Standard Cell Potential Of Al Decode electrochemistry concepts with ease and. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Interpret electrode potentials in terms of relative oxidant and reductant. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Discover the spontaneity of redox reactions using our. Standard Cell Potential Of Al.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Of Al This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Decode. Standard Cell Potential Of Al.
From www.slideserve.com
PPT Chemistry 1011 PowerPoint Presentation, free download ID5404690 Standard Cell Potential Of Al Interpret electrode potentials in terms of relative oxidant and reductant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Decode electrochemistry concepts with ease and. Discover the spontaneity of redox reactions using our standard cell potential calculator. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids. Standard Cell Potential Of Al.
From www.numerade.com
SOLVED Use tabulated standard electrode potentials to calculate the Standard Cell Potential Of Al The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Only the difference between the potentials of two electrodes can be measured. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Interpret electrode potentials. Standard Cell Potential Of Al.
From chemistrynotes.com
Cell Potential Calculations and Line Notation Standard Cell Potential Of Al Describe and relate the definitions of electrode and cell potentials. Discover the spontaneity of redox reactions using our standard cell potential calculator. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Decode electrochemistry concepts with ease and. The standard cell potential (e°cell) is measured at. Standard Cell Potential Of Al.
From www.researchgate.net
Values of the standard potentials of cerium redox systems in anodic Standard Cell Potential Of Al The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and. Standard Cell Potential Of Al.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 Standard Cell Potential Of Al Decode electrochemistry concepts with ease and. Only the difference between the potentials of two electrodes can be measured. Describe and relate the definitions of electrode and cell potentials. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: This. Standard Cell Potential Of Al.
From notes.pegas.is
Types of Reactions Pega's Notes Standard Cell Potential Of Al Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard. Standard Cell Potential Of Al.