Is Delta H Always Negative . Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. Similarly δs could be either positive or negative. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. Technically, the o in the symbol should have. A negative δh means that heat flows from a system to its surroundings; The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). A positive δh means that heat flows into a. A negative deltah_f^o indicates that the formation of a compound is.
from www.chegg.com
Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. A negative deltah_f^o indicates that the formation of a compound is. Similarly δs could be either positive or negative. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. A positive δh means that heat flows into a. The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. Technically, the o in the symbol should have. A negative δh means that heat flows from a system to its surroundings; Δh could be negative (an exothermic reaction) or positive (an endothermic reaction).
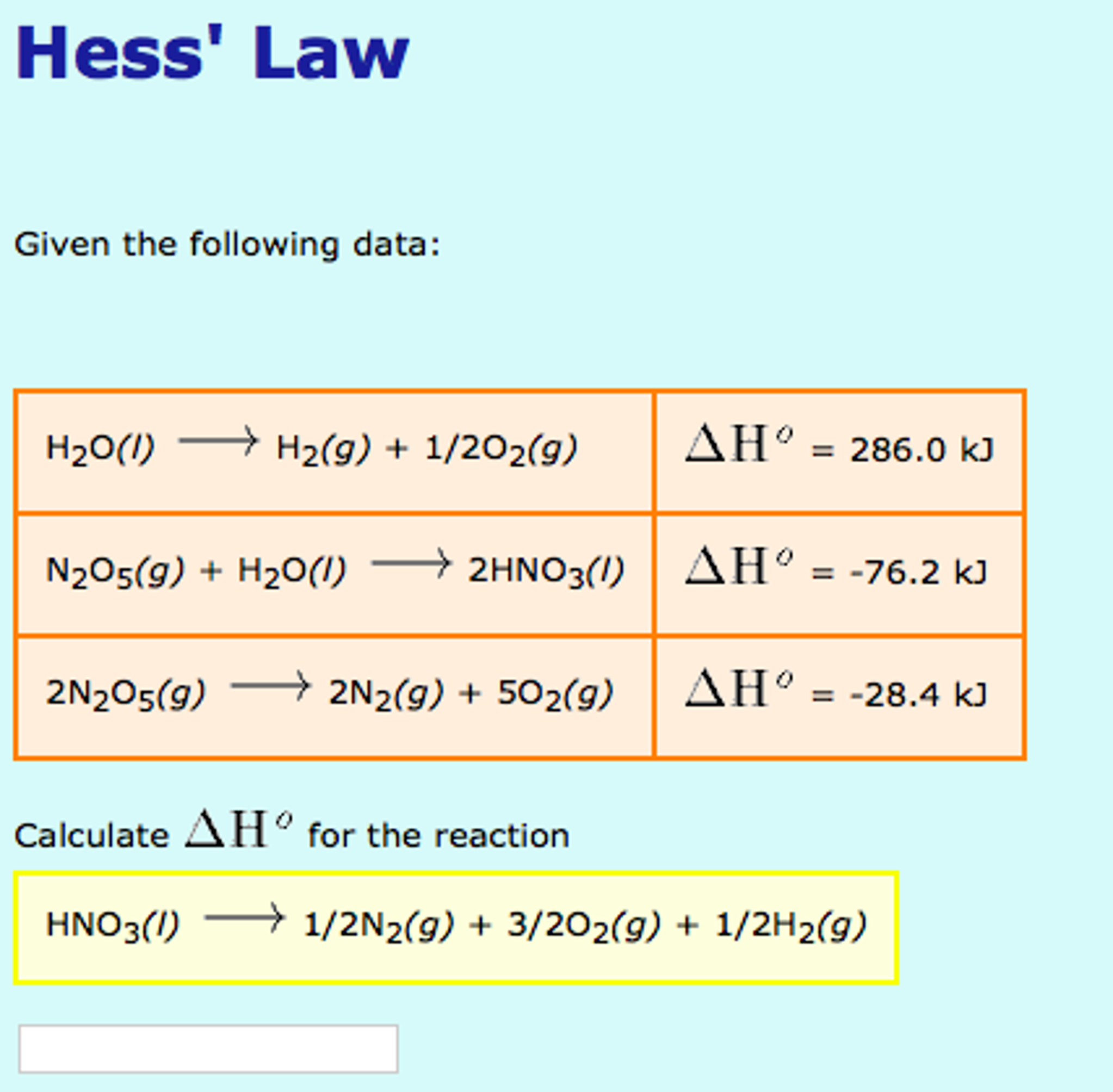
Solved Given the following data Calculate delta H^degree
Is Delta H Always Negative Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. A positive δh means that heat flows into a. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. A negative deltah_f^o indicates that the formation of a compound is. A negative δh means that heat flows from a system to its surroundings; Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. Similarly δs could be either positive or negative. Technically, the o in the symbol should have.
From www.youtube.com
15.2 Effect of ΔH, ΔS and T on the spontaneity of a reaction. (HL Is Delta H Always Negative A positive δh means that heat flows into a. Similarly δs could be either positive or negative. Technically, the o in the symbol should have. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium. Is Delta H Always Negative.
From cherries-everwhere.blogspot.com
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere Is Delta H Always Negative A negative δh means that heat flows from a system to its surroundings; Technically, the o in the symbol should have. A negative deltah_f^o indicates that the formation of a compound is. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). If heat flows from a system to its surroundings, the enthalpy of the system decreases, so. Is Delta H Always Negative.
From www.chegg.com
Solved For the given processes, determine if delta H^0_rxn Is Delta H Always Negative A negative deltah_f^o indicates that the formation of a compound is. Similarly δs could be either positive or negative. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. Technically, the o in the symbol should have. A positive δh means that heat flows into a. A negative δh means that. Is Delta H Always Negative.
From www.numerade.com
SOLVEDUse the symbols δ^+ and δ^ to show the direction of the Is Delta H Always Negative Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. A positive δh means that heat flows into a. If heat flows from. Is Delta H Always Negative.
From www.numerade.com
SOLVEDSuppose a reaction has a negative \Delta H^{\circ} and a Is Delta H Always Negative Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). #\delta h>0, \delta s>0# the enthalpy change is greater than zero because. Is Delta H Always Negative.
From www.chegg.com
Solved Consider a reaction that has a negative Delta H and a Is Delta H Always Negative Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). Technically, the o in the symbol should have. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. Similarly δs could be either positive or negative. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta. Is Delta H Always Negative.
From www.thetechedvocate.org
How to calculate deltah The Tech Edvocate Is Delta H Always Negative Technically, the o in the symbol should have. A negative deltah_f^o indicates that the formation of a compound is. Similarly δs could be either positive or negative. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. The symbol for a standard enthalpy change is δh°, read as delta h. Is Delta H Always Negative.
From socratic.org
How will temperature affect the spontaneity of a reaction with positive Is Delta H Always Negative Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). Technically, the o in the symbol should have. A negative deltah_f^o indicates that the formation of a compound is. A negative δh means that heat flows from a system to its surroundings; Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative.. Is Delta H Always Negative.
From www.expii.com
Spontaneous and Nonspontaneous Reactions — Overview Expii Is Delta H Always Negative Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. A positive δh means that heat flows into a. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so. Is Delta H Always Negative.
From www.chemistrysteps.com
Energy and Organic Chemistry Reactions Chemistry Steps Is Delta H Always Negative If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. Technically, the o in the symbol should have. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). A negative deltah_f^o indicates that the formation of a compound is. Combustion reactions are exothermic so the value for the. Is Delta H Always Negative.
From www.youtube.com
18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H Is Delta H Always Negative Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. A negative δh means that heat flows from a system to its surroundings; Similarly δs could be either positive or negative. A positive δh means that heat flows into a. Technically, the o in the symbol should have. If heat flows from a system. Is Delta H Always Negative.
From www.chegg.com
Solved Consider a reaction that has a negative delta H and a Is Delta H Always Negative A negative δh means that heat flows from a system to its surroundings; A positive δh means that heat flows into a. The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so. Is Delta H Always Negative.
From mavink.com
Delta H Reaction Formula Is Delta H Always Negative The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). A negative deltah_f^o indicates that the formation. Is Delta H Always Negative.
From www.virtualhomeschoolgroup.org
Study Notes Is Delta H Always Negative #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. A positive δh. Is Delta H Always Negative.
From general.chemistrysteps.com
The Effect of 𝚫H, 𝚫S, and T on 𝚫G Spontaneity Chemistry Steps Is Delta H Always Negative #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. A negative δh means that heat flows from a system to its surroundings; Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). A negative deltah_f^o indicates that the formation of a compound is. If heat flows from a. Is Delta H Always Negative.
From byjus.com
How when delta H is negative solubility decreases with temperature Is Delta H Always Negative Similarly δs could be either positive or negative. A negative deltah_f^o indicates that the formation of a compound is. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. A negative δh means that heat. Is Delta H Always Negative.
From cherries-everwhere.blogspot.com
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere Is Delta H Always Negative A positive δh means that heat flows into a. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). Technically, the o in the symbol should have. Combustion reactions are exothermic so the value for the enthalpy change. Is Delta H Always Negative.
From www.reddit.com
Help w entropy q? I get why there’s a positive delta S, but not really Is Delta H Always Negative The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). Technically, the o in the symbol should have. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. If heat. Is Delta H Always Negative.
From cherries-everwhere.blogspot.com
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere Is Delta H Always Negative #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. A negative deltah_f^o indicates that the formation of a compound is. A positive δh means that heat flows into. Is Delta H Always Negative.
From www.youtube.com
Free Energy and Predicting Spontaneous Reactions with H and S (Pt 6 Is Delta H Always Negative Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. A negative δh means that heat flows from a system to its surroundings; #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. A negative deltah_f^o indicates that the formation of a compound is. Technically,. Is Delta H Always Negative.
From www.youtube.com
5.1 Delta Hf and Delta Hc calculations [SL IB Chemistry] YouTube Is Delta H Always Negative A negative deltah_f^o indicates that the formation of a compound is. A positive δh means that heat flows into a. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). A negative δh means that heat flows from a system to its surroundings; If heat flows from a system to its surroundings, the enthalpy of the system decreases,. Is Delta H Always Negative.
From www.chegg.com
Solved Given the following data Calculate delta H^degree Is Delta H Always Negative A negative deltah_f^o indicates that the formation of a compound is. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). A positive δh means that heat flows into a. Similarly δs could be either positive or negative. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative.. Is Delta H Always Negative.
From general.chemistrysteps.com
The Effect of 𝚫H, 𝚫S, and T on 𝚫G Spontaneity Chemistry Steps Is Delta H Always Negative A negative δh means that heat flows from a system to its surroundings; The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. Similarly δs could be either positive or negative. Δh. Is Delta H Always Negative.
From www.numerade.com
SOLVEDA reaction for which ΔH and ΔS are both negative is (a Is Delta H Always Negative Technically, the o in the symbol should have. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. A negative deltah_f^o indicates that the formation of a compound is. A negative δh means that heat flows from a system to its surroundings; The symbol for a standard enthalpy change is δh°,. Is Delta H Always Negative.
From flexbooks.ck12.org
CK12Foundation Is Delta H Always Negative A positive δh means that heat flows into a. Similarly δs could be either positive or negative. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. Technically, the o in the symbol should. Is Delta H Always Negative.
From www.numerade.com
SOLVED Using the Gibbs Free Energy Formula, if a reaction is Is Delta H Always Negative Technically, the o in the symbol should have. The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid. Is Delta H Always Negative.
From www.chegg.com
Solved What combinations of positive or negative Delta H and Is Delta H Always Negative The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. Similarly δs could be either positive or negative. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). Technically, the o in the symbol should have. #\delta h>0, \delta s>0# the enthalpy change is greater than. Is Delta H Always Negative.
From www.chegg.com
Solved Predict the signs of Delta H degree, Delta S degree, Is Delta H Always Negative Technically, the o in the symbol should have. A negative deltah_f^o indicates that the formation of a compound is. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. A positive δh. Is Delta H Always Negative.
From www.toppr.com
For which of the following reaction Delta H is equal to Delta u? Is Delta H Always Negative Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). Technically, the o in the symbol should have. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is negative. A positive δh means. Is Delta H Always Negative.
From www.youtube.com
Change in enthalpy can be positive or negative Reactions meriSTEM Is Delta H Always Negative The symbol for a standard enthalpy change is δh°, read as delta h standard or, perhaps more commonly, as delta h nought. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). A negative δh means that heat flows from a system to its surroundings; Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)). Is Delta H Always Negative.
From www.youtube.com
Free energy (using delta H & delta S) YouTube Is Delta H Always Negative A negative deltah_f^o indicates that the formation of a compound is. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. Technically, the o in the symbol should have. A negative δh means that heat. Is Delta H Always Negative.
From www.slideserve.com
PPT Enthalpy, Entropy and Gibbs Law of Free Energy PowerPoint Is Delta H Always Negative Combustion reactions are exothermic so the value for the enthalpy change (\(\delta h\)) is always negative. A negative deltah_f^o indicates that the formation of a compound is. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. The symbol for a standard enthalpy change is δh°, read as delta h standard. Is Delta H Always Negative.
From www.chegg.com
Solved What is Delta H and Delta s, positive or negative Is Delta H Always Negative #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. Similarly δs could be either positive or negative. A negative deltah_f^o indicates that the formation of a compound is. A positive δh means that heat flows into a. Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). A. Is Delta H Always Negative.
From byjus.com
Dimerisation of NO2 is an exothermic reaction. if enthalpy of reaction Is Delta H Always Negative Δh could be negative (an exothermic reaction) or positive (an endothermic reaction). Similarly δs could be either positive or negative. A negative δh means that heat flows from a system to its surroundings; Technically, the o in the symbol should have. If heat flows from a system to its surroundings, the enthalpy of the system decreases, so δ hrxn is. Is Delta H Always Negative.
From www.numerade.com
SOLVED\Delta H for a reaction is negative. Compa… Is Delta H Always Negative Technically, the o in the symbol should have. #\delta h>0, \delta s>0# the enthalpy change is greater than zero because the solid calcium carbonate is more inherently. A positive δh means that heat flows into a. A negative deltah_f^o indicates that the formation of a compound is. A negative δh means that heat flows from a system to its surroundings;. Is Delta H Always Negative.