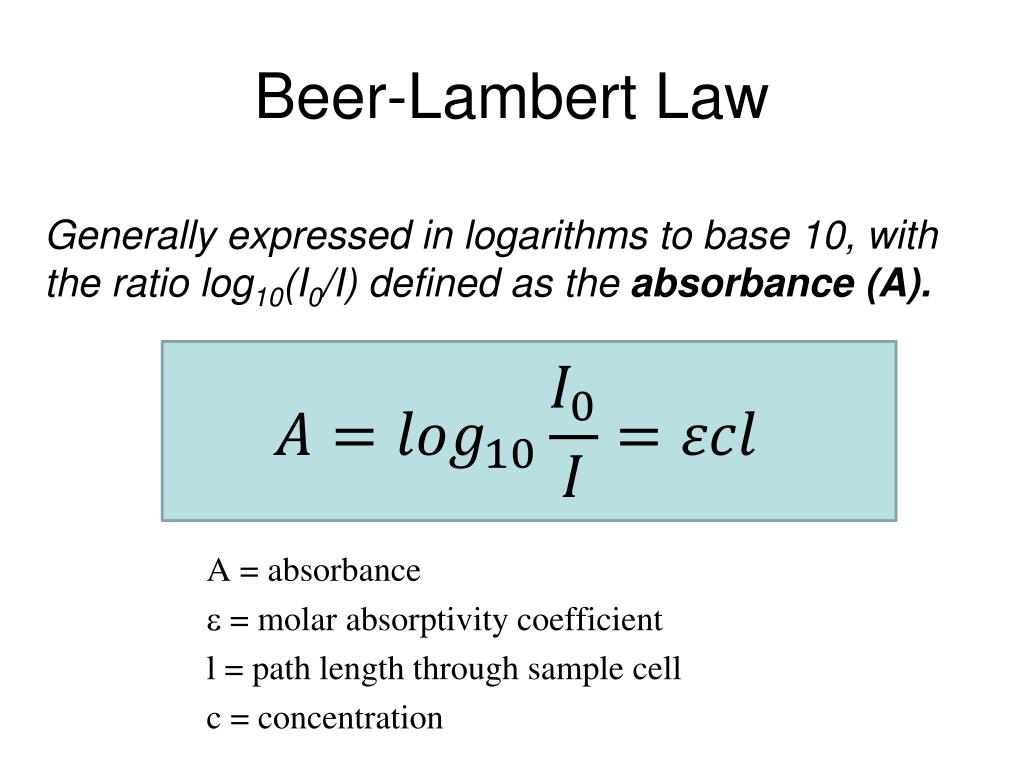
Beer Lambert Law Molar Extinction Coefficient . Take the antilog of the value obtained in. A = εcl (4) (4) a = ε c l. Subtract the absorbance value from the number 2. (1) where ℓ is the. $$ a = \epsilon c\ell $$. A is the absorbance (dimensionless); A= εcl a = ε c l. To calculate transmittance from absorbance, we need to follow the given steps:
from www.slideserve.com
A= εcl a = ε c l. Take the antilog of the value obtained in. Subtract the absorbance value from the number 2. (1) where ℓ is the. A is the absorbance (dimensionless); A = εcl (4) (4) a = ε c l. $$ a = \epsilon c\ell $$. To calculate transmittance from absorbance, we need to follow the given steps:
PPT Analytical Chemistry PowerPoint Presentation, free download ID
Beer Lambert Law Molar Extinction Coefficient A= εcl a = ε c l. A = εcl (4) (4) a = ε c l. (1) where ℓ is the. Subtract the absorbance value from the number 2. A= εcl a = ε c l. $$ a = \epsilon c\ell $$. A is the absorbance (dimensionless); Take the antilog of the value obtained in. To calculate transmittance from absorbance, we need to follow the given steps:
From www.researchgate.net
Figure S8. Determination of the molar extinction coefficient of (3PS) 2 Beer Lambert Law Molar Extinction Coefficient $$ a = \epsilon c\ell $$. A is the absorbance (dimensionless); A= εcl a = ε c l. To calculate transmittance from absorbance, we need to follow the given steps: (1) where ℓ is the. Subtract the absorbance value from the number 2. Take the antilog of the value obtained in. A = εcl (4) (4) a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law Molar Extinction Coefficient Subtract the absorbance value from the number 2. To calculate transmittance from absorbance, we need to follow the given steps: A is the absorbance (dimensionless); $$ a = \epsilon c\ell $$. (1) where ℓ is the. A = εcl (4) (4) a = ε c l. Take the antilog of the value obtained in. A= εcl a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From stuff.iorodeo.com
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User Beer Lambert Law Molar Extinction Coefficient Take the antilog of the value obtained in. (1) where ℓ is the. Subtract the absorbance value from the number 2. A= εcl a = ε c l. A = εcl (4) (4) a = ε c l. $$ a = \epsilon c\ell $$. A is the absorbance (dimensionless); To calculate transmittance from absorbance, we need to follow the given. Beer Lambert Law Molar Extinction Coefficient.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Molar Extinction Coefficient (1) where ℓ is the. A= εcl a = ε c l. Subtract the absorbance value from the number 2. Take the antilog of the value obtained in. $$ a = \epsilon c\ell $$. A is the absorbance (dimensionless); To calculate transmittance from absorbance, we need to follow the given steps: A = εcl (4) (4) a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Beer Lambert Law Molar Extinction Coefficient A= εcl a = ε c l. To calculate transmittance from absorbance, we need to follow the given steps: A = εcl (4) (4) a = ε c l. $$ a = \epsilon c\ell $$. Subtract the absorbance value from the number 2. (1) where ℓ is the. Take the antilog of the value obtained in. A is the absorbance. Beer Lambert Law Molar Extinction Coefficient.
From www.slideserve.com
PPT Spectrophotometric Determination of Iron Using 1,10 Beer Lambert Law Molar Extinction Coefficient Take the antilog of the value obtained in. (1) where ℓ is the. To calculate transmittance from absorbance, we need to follow the given steps: A is the absorbance (dimensionless); $$ a = \epsilon c\ell $$. Subtract the absorbance value from the number 2. A = εcl (4) (4) a = ε c l. A= εcl a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From www.youtube.com
Beer Lambert Law, Molar Extinction Coefficient, Spectrophotometry YouTube Beer Lambert Law Molar Extinction Coefficient $$ a = \epsilon c\ell $$. Subtract the absorbance value from the number 2. (1) where ℓ is the. A= εcl a = ε c l. A = εcl (4) (4) a = ε c l. Take the antilog of the value obtained in. A is the absorbance (dimensionless); To calculate transmittance from absorbance, we need to follow the given. Beer Lambert Law Molar Extinction Coefficient.
From pt.slideshare.net
Molar extinction coefficient Beer Lambert Law Molar Extinction Coefficient $$ a = \epsilon c\ell $$. Subtract the absorbance value from the number 2. To calculate transmittance from absorbance, we need to follow the given steps: (1) where ℓ is the. A= εcl a = ε c l. Take the antilog of the value obtained in. A = εcl (4) (4) a = ε c l. A is the absorbance. Beer Lambert Law Molar Extinction Coefficient.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Molar Extinction Coefficient A = εcl (4) (4) a = ε c l. To calculate transmittance from absorbance, we need to follow the given steps: Subtract the absorbance value from the number 2. A= εcl a = ε c l. Take the antilog of the value obtained in. (1) where ℓ is the. $$ a = \epsilon c\ell $$. A is the absorbance. Beer Lambert Law Molar Extinction Coefficient.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer Lambert Law Molar Extinction Coefficient $$ a = \epsilon c\ell $$. A = εcl (4) (4) a = ε c l. To calculate transmittance from absorbance, we need to follow the given steps: A= εcl a = ε c l. Take the antilog of the value obtained in. Subtract the absorbance value from the number 2. A is the absorbance (dimensionless); (1) where ℓ is. Beer Lambert Law Molar Extinction Coefficient.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law Molar Extinction Coefficient (1) where ℓ is the. Take the antilog of the value obtained in. $$ a = \epsilon c\ell $$. A = εcl (4) (4) a = ε c l. Subtract the absorbance value from the number 2. To calculate transmittance from absorbance, we need to follow the given steps: A= εcl a = ε c l. A is the absorbance. Beer Lambert Law Molar Extinction Coefficient.
From www.researchgate.net
(Left axis) Molar extinction coefficient, ϵ A (λ), of a 10 −5 M QDs Beer Lambert Law Molar Extinction Coefficient A= εcl a = ε c l. Take the antilog of the value obtained in. To calculate transmittance from absorbance, we need to follow the given steps: (1) where ℓ is the. $$ a = \epsilon c\ell $$. A is the absorbance (dimensionless); A = εcl (4) (4) a = ε c l. Subtract the absorbance value from the number. Beer Lambert Law Molar Extinction Coefficient.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer Lambert Law Molar Extinction Coefficient Take the antilog of the value obtained in. A= εcl a = ε c l. Subtract the absorbance value from the number 2. To calculate transmittance from absorbance, we need to follow the given steps: (1) where ℓ is the. A is the absorbance (dimensionless); $$ a = \epsilon c\ell $$. A = εcl (4) (4) a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From www.researchgate.net
Determination of molar extinction coefficient (ε) for 11 nm CsPbBr3 Beer Lambert Law Molar Extinction Coefficient Take the antilog of the value obtained in. (1) where ℓ is the. To calculate transmittance from absorbance, we need to follow the given steps: Subtract the absorbance value from the number 2. A is the absorbance (dimensionless); $$ a = \epsilon c\ell $$. A= εcl a = ε c l. A = εcl (4) (4) a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From www.youtube.com
How to calculate molar extinction coefficient in Origin YouTube Beer Lambert Law Molar Extinction Coefficient A is the absorbance (dimensionless); (1) where ℓ is the. A = εcl (4) (4) a = ε c l. A= εcl a = ε c l. Subtract the absorbance value from the number 2. $$ a = \epsilon c\ell $$. To calculate transmittance from absorbance, we need to follow the given steps: Take the antilog of the value obtained. Beer Lambert Law Molar Extinction Coefficient.
From www.numerade.com
SOLVED 3. What is the difference between a single beam and double beam Beer Lambert Law Molar Extinction Coefficient A= εcl a = ε c l. To calculate transmittance from absorbance, we need to follow the given steps: A = εcl (4) (4) a = ε c l. (1) where ℓ is the. Take the antilog of the value obtained in. Subtract the absorbance value from the number 2. A is the absorbance (dimensionless); $$ a = \epsilon c\ell. Beer Lambert Law Molar Extinction Coefficient.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer Lambert Law Molar Extinction Coefficient Subtract the absorbance value from the number 2. A = εcl (4) (4) a = ε c l. Take the antilog of the value obtained in. To calculate transmittance from absorbance, we need to follow the given steps: (1) where ℓ is the. $$ a = \epsilon c\ell $$. A is the absorbance (dimensionless); A= εcl a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Beer Lambert Law Molar Extinction Coefficient Take the antilog of the value obtained in. (1) where ℓ is the. Subtract the absorbance value from the number 2. A= εcl a = ε c l. A = εcl (4) (4) a = ε c l. To calculate transmittance from absorbance, we need to follow the given steps: $$ a = \epsilon c\ell $$. A is the absorbance. Beer Lambert Law Molar Extinction Coefficient.
From dokumen.tips
(PDF) Determination of the Molar Extinction Coefficient of Beer Lambert Law Molar Extinction Coefficient (1) where ℓ is the. A= εcl a = ε c l. Take the antilog of the value obtained in. To calculate transmittance from absorbance, we need to follow the given steps: A is the absorbance (dimensionless); $$ a = \epsilon c\ell $$. Subtract the absorbance value from the number 2. A = εcl (4) (4) a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer Lambert Law Molar Extinction Coefficient (1) where ℓ is the. Subtract the absorbance value from the number 2. A = εcl (4) (4) a = ε c l. A is the absorbance (dimensionless); To calculate transmittance from absorbance, we need to follow the given steps: Take the antilog of the value obtained in. $$ a = \epsilon c\ell $$. A= εcl a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From www.researchgate.net
Application of the BeerLambert law to blue and NIR light Comparison of Beer Lambert Law Molar Extinction Coefficient A = εcl (4) (4) a = ε c l. (1) where ℓ is the. A is the absorbance (dimensionless); Subtract the absorbance value from the number 2. $$ a = \epsilon c\ell $$. Take the antilog of the value obtained in. To calculate transmittance from absorbance, we need to follow the given steps: A= εcl a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From www.numerade.com
SOLVED Write down an expression for the BeerLambert Law in terms of Beer Lambert Law Molar Extinction Coefficient A= εcl a = ε c l. A = εcl (4) (4) a = ε c l. Subtract the absorbance value from the number 2. Take the antilog of the value obtained in. (1) where ℓ is the. To calculate transmittance from absorbance, we need to follow the given steps: $$ a = \epsilon c\ell $$. A is the absorbance. Beer Lambert Law Molar Extinction Coefficient.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer Lambert Law Molar Extinction Coefficient (1) where ℓ is the. Take the antilog of the value obtained in. Subtract the absorbance value from the number 2. A is the absorbance (dimensionless); To calculate transmittance from absorbance, we need to follow the given steps: $$ a = \epsilon c\ell $$. A= εcl a = ε c l. A = εcl (4) (4) a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer Lambert Law Molar Extinction Coefficient $$ a = \epsilon c\ell $$. Take the antilog of the value obtained in. Subtract the absorbance value from the number 2. A = εcl (4) (4) a = ε c l. A is the absorbance (dimensionless); A= εcl a = ε c l. To calculate transmittance from absorbance, we need to follow the given steps: (1) where ℓ is. Beer Lambert Law Molar Extinction Coefficient.
From www.slideserve.com
PPT Absorption and Scattering PowerPoint Presentation, free download Beer Lambert Law Molar Extinction Coefficient Subtract the absorbance value from the number 2. (1) where ℓ is the. To calculate transmittance from absorbance, we need to follow the given steps: A = εcl (4) (4) a = ε c l. Take the antilog of the value obtained in. A= εcl a = ε c l. A is the absorbance (dimensionless); $$ a = \epsilon c\ell. Beer Lambert Law Molar Extinction Coefficient.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Beer Lambert Law Molar Extinction Coefficient Take the antilog of the value obtained in. (1) where ℓ is the. A= εcl a = ε c l. Subtract the absorbance value from the number 2. To calculate transmittance from absorbance, we need to follow the given steps: A is the absorbance (dimensionless); A = εcl (4) (4) a = ε c l. $$ a = \epsilon c\ell. Beer Lambert Law Molar Extinction Coefficient.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Molar Extinction Coefficient $$ a = \epsilon c\ell $$. Take the antilog of the value obtained in. (1) where ℓ is the. A = εcl (4) (4) a = ε c l. A is the absorbance (dimensionless); A= εcl a = ε c l. Subtract the absorbance value from the number 2. To calculate transmittance from absorbance, we need to follow the given. Beer Lambert Law Molar Extinction Coefficient.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Molar Extinction Coefficient A = εcl (4) (4) a = ε c l. $$ a = \epsilon c\ell $$. To calculate transmittance from absorbance, we need to follow the given steps: Take the antilog of the value obtained in. (1) where ℓ is the. A is the absorbance (dimensionless); Subtract the absorbance value from the number 2. A= εcl a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From pt.slideshare.net
Molar extinction coefficient Beer Lambert Law Molar Extinction Coefficient A is the absorbance (dimensionless); $$ a = \epsilon c\ell $$. Subtract the absorbance value from the number 2. To calculate transmittance from absorbance, we need to follow the given steps: Take the antilog of the value obtained in. A= εcl a = ε c l. A = εcl (4) (4) a = ε c l. (1) where ℓ is. Beer Lambert Law Molar Extinction Coefficient.
From www.numerade.com
Data Analysis Using the absorbance data and BeerLambert's law, the Beer Lambert Law Molar Extinction Coefficient $$ a = \epsilon c\ell $$. Subtract the absorbance value from the number 2. A = εcl (4) (4) a = ε c l. To calculate transmittance from absorbance, we need to follow the given steps: Take the antilog of the value obtained in. A= εcl a = ε c l. (1) where ℓ is the. A is the absorbance. Beer Lambert Law Molar Extinction Coefficient.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law Beer Lambert Law Molar Extinction Coefficient (1) where ℓ is the. $$ a = \epsilon c\ell $$. To calculate transmittance from absorbance, we need to follow the given steps: Take the antilog of the value obtained in. Subtract the absorbance value from the number 2. A = εcl (4) (4) a = ε c l. A is the absorbance (dimensionless); A= εcl a = ε c. Beer Lambert Law Molar Extinction Coefficient.
From mavink.com
Molar Absorptivity Chart Beer Lambert Law Molar Extinction Coefficient $$ a = \epsilon c\ell $$. A is the absorbance (dimensionless); Subtract the absorbance value from the number 2. Take the antilog of the value obtained in. A = εcl (4) (4) a = ε c l. (1) where ℓ is the. A= εcl a = ε c l. To calculate transmittance from absorbance, we need to follow the given. Beer Lambert Law Molar Extinction Coefficient.
From www.numerade.com
SOLVED The protein bovine serum albumin (BSA), has a molecular weight Beer Lambert Law Molar Extinction Coefficient Take the antilog of the value obtained in. $$ a = \epsilon c\ell $$. A = εcl (4) (4) a = ε c l. A is the absorbance (dimensionless); A= εcl a = ε c l. (1) where ℓ is the. Subtract the absorbance value from the number 2. To calculate transmittance from absorbance, we need to follow the given. Beer Lambert Law Molar Extinction Coefficient.
From www.slideserve.com
PPT Exp 14B Determining an Equilibrium Constant PowerPoint Beer Lambert Law Molar Extinction Coefficient A = εcl (4) (4) a = ε c l. Take the antilog of the value obtained in. To calculate transmittance from absorbance, we need to follow the given steps: A is the absorbance (dimensionless); (1) where ℓ is the. Subtract the absorbance value from the number 2. A= εcl a = ε c l. $$ a = \epsilon c\ell. Beer Lambert Law Molar Extinction Coefficient.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law Molar Extinction Coefficient Subtract the absorbance value from the number 2. A is the absorbance (dimensionless); To calculate transmittance from absorbance, we need to follow the given steps: (1) where ℓ is the. A = εcl (4) (4) a = ε c l. $$ a = \epsilon c\ell $$. A= εcl a = ε c l. Take the antilog of the value obtained. Beer Lambert Law Molar Extinction Coefficient.