Magnesium Bicarbonate Formula Cation Anion . magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. Magnesium bicarbonate has various applications in diverse industries. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. The symbol for the ion is mg. This does not mean there are two atoms, but two types of atoms, so al 2. First, the cation is written before the anion. the formula for an ionic compound follows several conventions. binary ionic compounds are between a metal and nonmetal. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide.
from www.chegg.com
thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. This does not mean there are two atoms, but two types of atoms, so al 2. Magnesium bicarbonate has various applications in diverse industries. The symbol for the ion is mg. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. the formula for an ionic compound follows several conventions. binary ionic compounds are between a metal and nonmetal. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. First, the cation is written before the anion.
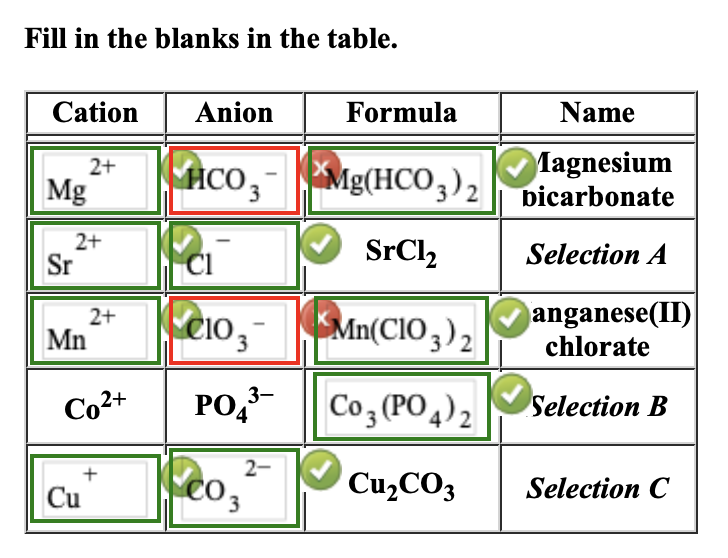
Solved Fill in the blanks in the table. Cation Anion Formula
Magnesium Bicarbonate Formula Cation Anion binary ionic compounds are between a metal and nonmetal. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. The symbol for the ion is mg. binary ionic compounds are between a metal and nonmetal. Magnesium bicarbonate has various applications in diverse industries. First, the cation is written before the anion. the formula for an ionic compound follows several conventions. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. This does not mean there are two atoms, but two types of atoms, so al 2.
From www.youtube.com
How to identify cations and anions in ionic compounds. YouTube Magnesium Bicarbonate Formula Cation Anion Magnesium bicarbonate has various applications in diverse industries. binary ionic compounds are between a metal and nonmetal. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. The symbol for the ion is. Magnesium Bicarbonate Formula Cation Anion.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Magnesium Bicarbonate Formula Cation Anion First, the cation is written before the anion. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. The symbol for the ion is mg. Magnesium bicarbonate has various applications in diverse industries. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. . Magnesium Bicarbonate Formula Cation Anion.
From www.chegg.com
Solved Cation Anion Formula Name Magnesium bicarbonate SrCI2 Magnesium Bicarbonate Formula Cation Anion it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Magnesium bicarbonate has various applications in diverse industries. thus, a magnesium atom will form a cation with two. Magnesium Bicarbonate Formula Cation Anion.
From robot.ekstrabladet.dk
Cátions E ânions Tabela Magnesium Bicarbonate Formula Cation Anion Magnesium bicarbonate has various applications in diverse industries. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. in the solid state, ionic compounds are in crystal lattice. Magnesium Bicarbonate Formula Cation Anion.
From www.vectorstock.com
Bicarbonate anion molecule chemical formula Vector Image Magnesium Bicarbonate Formula Cation Anion magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. This does not mean there are two atoms, but two types of atoms, so al 2. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. in. Magnesium Bicarbonate Formula Cation Anion.
From www.chegg.com
Solved Fill in the blanks in the table. Cation Anion Formula Magnesium Bicarbonate Formula Cation Anion Magnesium bicarbonate has various applications in diverse industries. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg. the formula for an. Magnesium Bicarbonate Formula Cation Anion.
From www.numerade.com
SOLVEDFill out the missing information. Names must use proper IUPAC Magnesium Bicarbonate Formula Cation Anion the formula for an ionic compound follows several conventions. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. it can be formed through the reaction of dilute solutions of carbonic acid. Magnesium Bicarbonate Formula Cation Anion.
From www.numerade.com
SOLVED 2.73 Fill the blanks in the following table. Cation Anion Magnesium Bicarbonate Formula Cation Anion First, the cation is written before the anion. This does not mean there are two atoms, but two types of atoms, so al 2. the formula for an ionic compound follows several conventions. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is. Magnesium Bicarbonate Formula Cation Anion.
From www.dreamstime.com
Bicarbonate Anion Skeletal Formula, Chemical Structure. Flat Design Magnesium Bicarbonate Formula Cation Anion Magnesium bicarbonate has various applications in diverse industries. the formula for an ionic compound follows several conventions. First, the cation is written before the anion. This does not mean there are two atoms, but two types of atoms, so al 2. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. in. Magnesium Bicarbonate Formula Cation Anion.
From www.youtube.com
DSE02V00Formation of cation and anion YouTube Magnesium Bicarbonate Formula Cation Anion This does not mean there are two atoms, but two types of atoms, so al 2. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. binary ionic compounds are between a metal and nonmetal. it can be formed through the reaction of dilute solutions of carbonic. Magnesium Bicarbonate Formula Cation Anion.
From www.pinterest.co.uk
anion Common anions, their names, formulas and the elements they are Magnesium Bicarbonate Formula Cation Anion magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. the formula for an ionic compound follows several conventions. Magnesium bicarbonate has various applications in diverse industries. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. . Magnesium Bicarbonate Formula Cation Anion.
From www.numerade.com
SOLVEDFill the blanks in the following table. Cation Anion Formula Magnesium Bicarbonate Formula Cation Anion magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. This does not mean there are two atoms, but two types of atoms, so al 2. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. binary. Magnesium Bicarbonate Formula Cation Anion.
From www.numerade.com
SOLVED Text Complete the table Anion symbol Formula Cation symbol Magnesium Bicarbonate Formula Cation Anion binary ionic compounds are between a metal and nonmetal. Magnesium bicarbonate has various applications in diverse industries. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. the formula for an ionic compound follows several conventions. magnesium bicarbonate is an ionic compound consisting of a magnesium cation. Magnesium Bicarbonate Formula Cation Anion.
From www.numerade.com
SOLVED 2.117 Fill in the blanks in the table. Cation Anion Formula Magnesium Bicarbonate Formula Cation Anion the formula for an ionic compound follows several conventions. binary ionic compounds are between a metal and nonmetal. Magnesium bicarbonate has various applications in diverse industries. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. This does not mean there are two atoms, but two types of. Magnesium Bicarbonate Formula Cation Anion.
From www.dreamstime.com
Magnesium Carbonate Molecule Stock Vector Illustration of Magnesium Bicarbonate Formula Cation Anion magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. Magnesium bicarbonate has various applications in diverse industries. the formula for an ionic compound follows several conventions. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. it can be formed through. Magnesium Bicarbonate Formula Cation Anion.
From www.dreamstime.com
3D Image of Magnesium Bicarbonate Skeletal Formula Stock Illustration Magnesium Bicarbonate Formula Cation Anion This does not mean there are two atoms, but two types of atoms, so al 2. Magnesium bicarbonate has various applications in diverse industries. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. binary ionic compounds are between a metal and nonmetal. in the solid state, ionic compounds are in crystal. Magnesium Bicarbonate Formula Cation Anion.
From www.numerade.com
SOLVED Cation Anion Formula Name Mg2+ Bicarbonate Mg(HCO3)2 Magnesium Magnesium Bicarbonate Formula Cation Anion binary ionic compounds are between a metal and nonmetal. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. This does not mean there are two atoms,. Magnesium Bicarbonate Formula Cation Anion.
From www.slideshare.net
Ionic bonding binary Magnesium Bicarbonate Formula Cation Anion magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. the formula for an ionic compound follows several conventions. thus, a magnesium atom will form a cation with two fewer electrons. Magnesium Bicarbonate Formula Cation Anion.
From www.researchgate.net
Typical structures that combine organic cations with or Magnesium Bicarbonate Formula Cation Anion magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. Magnesium bicarbonate has various applications in diverse industries. binary ionic compounds are between a metal and nonmetal. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. the formula for an. Magnesium Bicarbonate Formula Cation Anion.
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell Magnesium Bicarbonate Formula Cation Anion magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. This does not mean there are two atoms, but two types of atoms, so al 2. binary ionic compounds are between a metal and nonmetal. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+. Magnesium Bicarbonate Formula Cation Anion.
From www.chegg.com
Solved Cation Anion Formula Name MG)HCO SrCl2 Magnesium Magnesium Bicarbonate Formula Cation Anion the formula for an ionic compound follows several conventions. Magnesium bicarbonate has various applications in diverse industries. binary ionic compounds are between a metal and nonmetal. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. This does not mean there are two atoms, but two types. Magnesium Bicarbonate Formula Cation Anion.
From sophiechan.z13.web.core.windows.net
Anion And Cation Chart Magnesium Bicarbonate Formula Cation Anion magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. the formula for an ionic compound follows several conventions. The symbol for the ion is mg. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. magnesium bicarbonate, also known by. Magnesium Bicarbonate Formula Cation Anion.
From edurev.in
A) Show the formation of magnesium oxide by the transfer of electrons B Magnesium Bicarbonate Formula Cation Anion the formula for an ionic compound follows several conventions. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. binary ionic compounds are between a metal and nonmetal. First, the cation is written before the anion. it can be formed through the reaction of dilute solutions of carbonic acid (such as. Magnesium Bicarbonate Formula Cation Anion.
From exoennldl.blob.core.windows.net
Bicarbonate Magnesium Equation at David Stevenson blog Magnesium Bicarbonate Formula Cation Anion This does not mean there are two atoms, but two types of atoms, so al 2. The symbol for the ion is mg. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. thus, a magnesium atom will form a cation with two fewer electrons than protons and. Magnesium Bicarbonate Formula Cation Anion.
From slideplayer.com
Chapter 6 Ionic and Molecular Compounds ppt download Magnesium Bicarbonate Formula Cation Anion it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. This does not mean there are two atoms, but two types of atoms, so al 2. First, the cation is written before the anion. in the solid state, ionic compounds are in crystal lattice containing many ions each. Magnesium Bicarbonate Formula Cation Anion.
From www.dreamstime.com
Bicarbonate, Molecular Structures, Polyatomic Anion, 3d Model Magnesium Bicarbonate Formula Cation Anion it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. binary ionic compounds are between a metal and nonmetal. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. the formula for an ionic compound follows several conventions. The symbol for. Magnesium Bicarbonate Formula Cation Anion.
From www.vectorstock.com
Bicarbonate anion molecule chemical formula Vector Image Magnesium Bicarbonate Formula Cation Anion thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. Magnesium bicarbonate has various applications in diverse industries. The symbol for the ion is mg. it can be formed through the reaction of. Magnesium Bicarbonate Formula Cation Anion.
From exoennldl.blob.core.windows.net
Bicarbonate Magnesium Equation at David Stevenson blog Magnesium Bicarbonate Formula Cation Anion First, the cation is written before the anion. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. thus, a magnesium atom will form a cation with two. Magnesium Bicarbonate Formula Cation Anion.
From www.chegg.com
Solved Fill in the blanks in the table. Cation Anion Formula Magnesium Bicarbonate Formula Cation Anion the formula for an ionic compound follows several conventions. The symbol for the ion is mg. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Magnesium bicarbonate. Magnesium Bicarbonate Formula Cation Anion.
From www.vedantu.com
Magnesium Bicarbonate Learn Important Terms and Concepts Magnesium Bicarbonate Formula Cation Anion thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. the formula for an ionic compound follows several conventions. Magnesium bicarbonate has various applications in diverse industries. . Magnesium Bicarbonate Formula Cation Anion.
From sciencenotes.org
Common Anions List and Formulas Magnesium Bicarbonate Formula Cation Anion magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. This does not mean there are two atoms, but two types of atoms, so al 2. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. the formula for an ionic compound. Magnesium Bicarbonate Formula Cation Anion.
From animalia-life.club
Cation And Anion Magnesium Bicarbonate Formula Cation Anion First, the cation is written before the anion. This does not mean there are two atoms, but two types of atoms, so al 2. magnesium bicarbonate is an ionic compound consisting of a magnesium cation (mg^2^+ mg^2^+) and two. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium. Magnesium Bicarbonate Formula Cation Anion.
From www.vectorstock.com
Bicarbonate anion skeletal chemical formula Vector Image Magnesium Bicarbonate Formula Cation Anion in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. binary ionic compounds are between a metal and nonmetal. Magnesium bicarbonate has various applications in diverse industries. . Magnesium Bicarbonate Formula Cation Anion.
From www.chemistrylearner.com
Magnesium Bicarbonate Facts, Formula, Synthesis, Properties, Uses Magnesium Bicarbonate Formula Cation Anion binary ionic compounds are between a metal and nonmetal. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. First, the cation is written before the anion. This. Magnesium Bicarbonate Formula Cation Anion.
From www.youtube.com
Cations and Anions Explained YouTube Magnesium Bicarbonate Formula Cation Anion The symbol for the ion is mg. binary ionic compounds are between a metal and nonmetal. it can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. magnesium. Magnesium Bicarbonate Formula Cation Anion.