Acetic Acid Limit As Per Ich . Pdes are defined as pharmaceutically acceptable intakes. Thus, the option 1 limit is 410 ppm. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. The permitted daily exposure to acetonitrile is 4.1 mg per day; See appendix 2 for additional The maximum administered daily mass of a drug pr. It recommends use of less toxic. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. Ich q3c provides permissible daily exposures (pdes) for residual solvents. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient.
from www.numerade.com
Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. The maximum administered daily mass of a drug pr. The permitted daily exposure to acetonitrile is 4.1 mg per day; Thus, the option 1 limit is 410 ppm. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. See appendix 2 for additional Pdes are defined as pharmaceutically acceptable intakes. Ich q3c provides permissible daily exposures (pdes) for residual solvents. This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient.
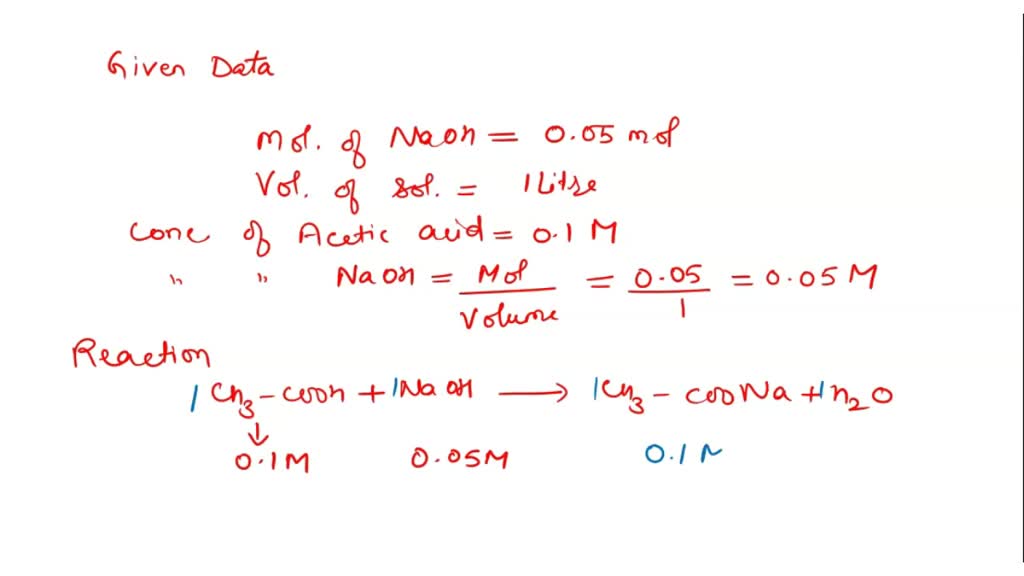
SOLVED Calculate the relative amount of acetic acid and acetate ion
Acetic Acid Limit As Per Ich The maximum administered daily mass of a drug pr. See appendix 2 for additional A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. Pdes are defined as pharmaceutically acceptable intakes. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. The maximum administered daily mass of a drug pr. Thus, the option 1 limit is 410 ppm. The permitted daily exposure to acetonitrile is 4.1 mg per day; It recommends use of less toxic. This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. Ich q3c provides permissible daily exposures (pdes) for residual solvents. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol.
From www.slideserve.com
PPT Equilibrium Acids and Bases PowerPoint Presentation, free Acetic Acid Limit As Per Ich Ich q3c provides permissible daily exposures (pdes) for residual solvents. Thus, the option 1 limit is 410 ppm. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. The maximum administered daily mass of a drug pr. It recommends use of less toxic. See appendix 2. Acetic Acid Limit As Per Ich.
From thegravityinstitute.com
Distinguish between formic acid and acetic acid The Gravity Acetic Acid Limit As Per Ich See appendix 2 for additional The maximum administered daily mass of a drug pr. Pdes are defined as pharmaceutically acceptable intakes. This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in. Acetic Acid Limit As Per Ich.
From collegedunia.com
Uses of Acetic Acid Definition, Structure, and Physical and Chemical Acetic Acid Limit As Per Ich The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. Ich q3c provides permissible daily exposures (pdes) for residual solvents. It recommends use of less toxic. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. See appendix 2 for. Acetic Acid Limit As Per Ich.
From www.coursehero.com
[Solved] Inall 2 Mass of acetic acid Density of acetic acid 1.00 g/mL Acetic Acid Limit As Per Ich Pdes are defined as pharmaceutically acceptable intakes. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. It recommends use of less toxic. The international council for harmonisation of. Acetic Acid Limit As Per Ich.
From abc13.com
Acetic acid explained What it is and how it's used ABC13 Houston Acetic Acid Limit As Per Ich The maximum administered daily mass of a drug pr. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. It recommends use of less toxic. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. The permitted daily exposure. Acetic Acid Limit As Per Ich.
From www.scribd.com
Threshold Limit Values (TLV) for All Chemicals Acetic Acid Benzene Acetic Acid Limit As Per Ich Thus, the option 1 limit is 410 ppm. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. See appendix 2 for additional A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. It recommends use of less toxic.. Acetic Acid Limit As Per Ich.
From qbd.co.kr
ICH Q3C 불순물 잔류용매 Acetic Acid Limit As Per Ich This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. The permitted daily exposure to acetonitrile is 4.1 mg per day; The maximum administered daily mass of a drug pr. A new solvent or revised limit that. Acetic Acid Limit As Per Ich.
From www.bartleby.com
Answered Acetic acid can be produced through the… bartleby Acetic Acid Limit As Per Ich See appendix 2 for additional This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. Thus, the option 1 limit is 410 ppm. It recommends use of less. Acetic Acid Limit As Per Ich.
From www.dreamstime.com
Acetic Acid, Structural Chemical Formula Stock Illustration Acetic Acid Limit As Per Ich A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. The maximum administered daily mass of a drug pr. Thus, the option 1 limit is 410 ppm. It recommends use of less toxic. This is the companion document for the international council for harmonisation of. Acetic Acid Limit As Per Ich.
From www.researchgate.net
SD Acid pretreatments a 2 acetic acid dose, b 3 acetic acid dose Acetic Acid Limit As Per Ich The permitted daily exposure to acetonitrile is 4.1 mg per day; A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. Pdes are defined as pharmaceutically acceptable intakes. The maximum administered daily mass of a drug pr. The international council for harmonisation of technical requirements. Acetic Acid Limit As Per Ich.
From www.researchgate.net
Acetic acid process, reaction conditions and chemical and bacterial Acetic Acid Limit As Per Ich Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. Ich q3c provides permissible daily exposures (pdes) for residual solvents. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. The permitted daily exposure to acetonitrile is 4.1 mg per. Acetic Acid Limit As Per Ich.
From www.compliancesigns.com
ANSI Acetic Acid Sign With GHS Symbol ADE37248 Acetic Acid Limit As Per Ich Thus, the option 1 limit is 410 ppm. Pdes are defined as pharmaceutically acceptable intakes. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. The maximum administered daily mass of a drug pr. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. Ich q3c provides permissible. Acetic Acid Limit As Per Ich.
From issuu.com
Acetic Acid and It’s Uses by tradeasiainternational Issuu Acetic Acid Limit As Per Ich This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. It recommends use. Acetic Acid Limit As Per Ich.
From thisnutrition.com
Acetic Topic This Nutrition Acetic Acid Limit As Per Ich A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct. Acetic Acid Limit As Per Ich.
From www.coffeechemistry.com
Acetic Acid Acetic Acid Limit As Per Ich The maximum administered daily mass of a drug pr. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. Thus, the option 1 limit is 410 ppm. It recommends use. Acetic Acid Limit As Per Ich.
From www.perstorp.com
C2 acetic acid infographic Acetic Acid Limit As Per Ich This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. See appendix 2 for additional The permitted daily exposure to acetonitrile is 4.1 mg per day; Pdes are defined as pharmaceutically acceptable intakes. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list. Acetic Acid Limit As Per Ich.
From marspedia.org
Deprecated Acetic Acid Limit As Per Ich The maximum administered daily mass of a drug pr. This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. Thus, the option 1 limit is 410 ppm. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. Pdes are. Acetic Acid Limit As Per Ich.
From www.dreamstime.com
Acetic Acid Hand Drawn Vector Formula Chemical Structure Lettering Blue Acetic Acid Limit As Per Ich Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. See appendix 2 for additional The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. The maximum administered daily mass of a drug pr. Thus, the option 1 limit is. Acetic Acid Limit As Per Ich.
From www.numerade.com
SOLVED Acetic anhydride is to be produced from acetone and acetic acid Acetic Acid Limit As Per Ich The permitted daily exposure to acetonitrile is 4.1 mg per day; Thus, the option 1 limit is 410 ppm. Ich q3c provides permissible daily exposures (pdes) for residual solvents. See appendix 2 for additional It recommends use of less toxic. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value. Acetic Acid Limit As Per Ich.
From mavink.com
Acetic Acid Phase Diagram Acetic Acid Limit As Per Ich It recommends use of less toxic. The maximum administered daily mass of a drug pr. The permitted daily exposure to acetonitrile is 4.1 mg per day; The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. This. Acetic Acid Limit As Per Ich.
From www.youtube.com
How to Write the Formula for Acetic acid YouTube Acetic Acid Limit As Per Ich See appendix 2 for additional The maximum administered daily mass of a drug pr. It recommends use of less toxic. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. Ich q3c provides permissible daily exposures (pdes) for residual solvents. This document recommends acceptable amounts. Acetic Acid Limit As Per Ich.
From testbook.com
Uses Of Acetic Acid Learn The Various Uses Of Acetic Acid in Detail Acetic Acid Limit As Per Ich The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. The permitted daily exposure to acetonitrile is 4.1 mg per day; Ich q3c provides permissible daily exposures (pdes) for residual solvents. Thus, the option 1 limit is 410. Acetic Acid Limit As Per Ich.
From pdfprof.com
acetic anhydride pka value Acetic Acid Limit As Per Ich It recommends use of less toxic. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. Pdes are defined as pharmaceutically acceptable intakes. Q3c(r7) correction for the pde and. Acetic Acid Limit As Per Ich.
From www.youtube.com
How to prepare 5 acetic acid solution ? YouTube Acetic Acid Limit As Per Ich The maximum administered daily mass of a drug pr. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. Ich q3c provides permissible daily exposures (pdes) for residual solvents. See appendix 2 for additional A new solvent or revised limit that has been approved through the. Acetic Acid Limit As Per Ich.
From www.tessshebaylo.com
Ionization Of Acetic Acid Equilibrium Equation Tessshebaylo Acetic Acid Limit As Per Ich This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. Thus, the option 1 limit is 410 ppm. Ich q3c provides permissible daily exposures (pdes) for residual solvents. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. Q3c(r7) correction for the pde and concentration limit for. Acetic Acid Limit As Per Ich.
From fr.wikidoc.org
Acetic acid wikidoc Acetic Acid Limit As Per Ich Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. See appendix 2 for additional The maximum administered daily mass of a drug pr. A new solvent or revised limit. Acetic Acid Limit As Per Ich.
From us.metoree.com
13 Acetic Acid Manufacturers in 2024 Metoree Acetic Acid Limit As Per Ich It recommends use of less toxic. The maximum administered daily mass of a drug pr. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. The permitted daily exposure to acetonitrile is 4.1 mg per day; Pdes are defined as pharmaceutically acceptable intakes. Thus, the option. Acetic Acid Limit As Per Ich.
From www.sciencephoto.com
Acetic acid molecule, illustration Stock Image F027/8027 Science Acetic Acid Limit As Per Ich The permitted daily exposure to acetonitrile is 4.1 mg per day; Pdes are defined as pharmaceutically acceptable intakes. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. See appendix 2 for additional This document recommends acceptable amounts for residual solvents in pharmaceuticals for the. Acetic Acid Limit As Per Ich.
From www.numerade.com
SOLVED Calculate the relative amount of acetic acid and acetate ion Acetic Acid Limit As Per Ich The permitted daily exposure to acetonitrile is 4.1 mg per day; This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. Ich q3c provides permissible daily exposures (pdes) for residual solvents. Thus, the option 1 limit is 410. Acetic Acid Limit As Per Ich.
From www.chegg.com
Solved An acetic acid molecule (CH3COOH) is shown Acetic Acid Limit As Per Ich The maximum administered daily mass of a drug pr. It recommends use of less toxic. Thus, the option 1 limit is 410 ppm. The permitted daily exposure to acetonitrile is 4.1 mg per day; This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. A new solvent or revised limit that has been approved. Acetic Acid Limit As Per Ich.
From www.researchgate.net
Class 2 solvents (2) Download Table Acetic Acid Limit As Per Ich Pdes are defined as pharmaceutically acceptable intakes. The maximum administered daily mass of a drug pr. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. This document. Acetic Acid Limit As Per Ich.
From www.alamy.com
Acetic Acid, Structural chemical formula on a white background Stock Acetic Acid Limit As Per Ich This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. Pdes are defined as pharmaceutically acceptable intakes. Thus, the option 1 limit is 410 ppm. The maximum administered daily mass. Acetic Acid Limit As Per Ich.
From www.vectorstock.com
Formula and model of acetic acid molecule Vector Image Acetic Acid Limit As Per Ich This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. Q3c(r7) correction for the pde and concentration limit for ethyleneglycol on table 2 page 6, as per the correct value calculated in pharmeuropa, vol. Pdes are defined as pharmaceutically acceptable intakes. Ich q3c provides permissible daily exposures (pdes) for residual solvents. This is the. Acetic Acid Limit As Per Ich.
From qbd.co.kr
ICH Q3C 불순물 잔류용매 Acetic Acid Limit As Per Ich The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) has the. This document recommends acceptable amounts for residual solvents in pharmaceuticals for the safety of the patient. The permitted daily exposure to acetonitrile is 4.1 mg per day; The maximum administered daily mass of a drug pr. It recommends use of less toxic. Ich q3c. Acetic Acid Limit As Per Ich.
From www.difference.wiki
Acetic Acid vs. Ethanoic Acid What’s the Difference? Acetic Acid Limit As Per Ich This is the companion document for the international council for harmonisation of technical requirements for pharmaceuticals for. It recommends use of less toxic. A new solvent or revised limit that has been approved through the ich process will be added to the appropriate list in this general chapter. The international council for harmonisation of technical requirements for pharmaceuticals for human. Acetic Acid Limit As Per Ich.