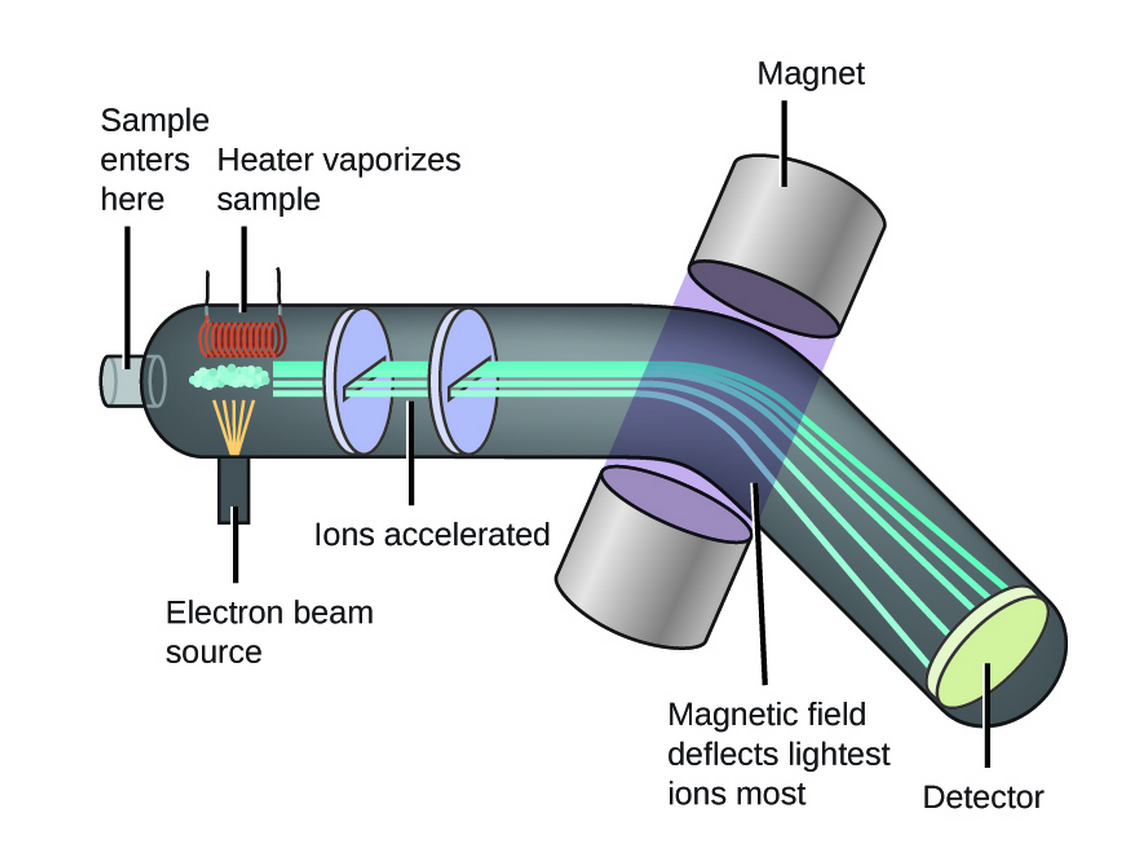
Isotopes And Mass Spectrometry . mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. There are many different types of ms instruments, but they all have the same three essential components. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding a proton. isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. the basics of a mass spectrometry experiment.
from ar.inspiredpencil.com
we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. the basics of a mass spectrometry experiment. There are many different types of ms instruments, but they all have the same three essential components. isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding a proton. The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions.
Spectrophotometer Labeled
Isotopes And Mass Spectrometry There are many different types of ms instruments, but they all have the same three essential components. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. There are many different types of ms instruments, but they all have the same three essential components. mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding a proton. the basics of a mass spectrometry experiment. we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig.
From blog.sepscience.com
The Role of Isotope Peak Intensities Obtained Using Mass Spectrometry Isotopes And Mass Spectrometry There are many different types of ms instruments, but they all have the same three essential components. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. The existence of isotopes was first observed by aston using a mass spectrometer to. Isotopes And Mass Spectrometry.
From www.slideserve.com
PPT Isotopes & Mass Spectrometry PowerPoint Presentation, free Isotopes And Mass Spectrometry First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding a proton. There are many different types of ms instruments, but they all have the same three essential components. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20. Isotopes And Mass Spectrometry.
From www.studypool.com
SOLUTION Isotopes and mass spectrometry Studypool Isotopes And Mass Spectrometry The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding a. Isotopes And Mass Spectrometry.
From www.thermofisher.com
DELTA™ Q Isotope Ratio Mass Spectrometer Isotopes And Mass Spectrometry isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. The existence of isotopes was first observed by aston using a mass. Isotopes And Mass Spectrometry.
From www.geology.pitt.edu
Isotope Ratio Mass Spectrometers Department of Geology and Isotopes And Mass Spectrometry When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. The existence of isotopes was first observed by aston using a. Isotopes And Mass Spectrometry.
From www.youtube.com
Mass Spectrometry Isotopes YouTube Isotopes And Mass Spectrometry mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an. Isotopes And Mass Spectrometry.
From www.researchgate.net
Diagram of the laser ablation isotope ratio mass spectrometry analysis Isotopes And Mass Spectrometry the basics of a mass spectrometry experiment. we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. The existence of isotopes was first observed by aston. Isotopes And Mass Spectrometry.
From wisc.pb.unizin.org
Isotopes, Atomic Mass, and Mass Spectrometry (M2Q3) UWMadison Isotopes And Mass Spectrometry The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass.. Isotopes And Mass Spectrometry.
From www.thermofisher.com
Isotope Ratio Mass Spectrometry (IRMS) Thermo Fisher Scientific US Isotopes And Mass Spectrometry we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. There are many different types of ms instruments, but they all have the same three essential components. isotopes are nuclides that differ. Isotopes And Mass Spectrometry.
From www.youtube.com
CLIPT Episode 3 How a Stable Isotope Ratio Mass Spectrometer works Isotopes And Mass Spectrometry First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding a proton. we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. When interpreting mass spectra it is important to remember that the relative atomic mass or. Isotopes And Mass Spectrometry.
From www.geology.pitt.edu
Isotope Ratio Mass Spectrometers Department of Geology and Isotopes And Mass Spectrometry the basics of a mass spectrometry experiment. mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. First, there is an ionization. Isotopes And Mass Spectrometry.
From mci.si.edu
Isotope Ratio Mass Spectrometry Facilities Museum Conservation Institute Isotopes And Mass Spectrometry First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding a proton. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. The existence of isotopes was first observed by aston using a mass. Isotopes And Mass Spectrometry.
From www.studypool.com
SOLUTION Isotopes and mass spectrometry Studypool Isotopes And Mass Spectrometry The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. we’ll examine how we use. Isotopes And Mass Spectrometry.
From www.researchgate.net
12 Thermo Scientific Delta V Isotope Ratio Mass Spectrometer and the Isotopes And Mass Spectrometry There are many different types of ms instruments, but they all have the same three essential components. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. isotopes are nuclides that differ from each other by their mass number rather. Isotopes And Mass Spectrometry.
From wisc.pb.unizin.org
Isotopes, Atomic Mass, and Mass Spectrometry (M2Q3) UWMadison Isotopes And Mass Spectrometry The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. we’ll. Isotopes And Mass Spectrometry.
From originanalytical.com
Stable Isotope Analysis Origin Analytical Isotopes And Mass Spectrometry mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. There are many different types of ms instruments, but they all have the same three essential components. the basics of a mass spectrometry experiment. The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions.. Isotopes And Mass Spectrometry.
From www.youtube.com
2A Isotopes and Mass Spectrometry Edexcel IAS Chemistry (Unit 1 Isotopes And Mass Spectrometry in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. we’ll examine how we use mass spectrometry. Isotopes And Mass Spectrometry.
From www.slideserve.com
PPT Mass Spectrometry (Mass Spec.) PowerPoint Presentation, free Isotopes And Mass Spectrometry The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element. Isotopes And Mass Spectrometry.
From wisc.pb.unizin.org
Isotopes, Atomic Mass, and Mass Spectrometry (M2Q3) UWMadison Isotopes And Mass Spectrometry isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. the basics of a mass spectrometry experiment. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. First, there is an. Isotopes And Mass Spectrometry.
From ar.inspiredpencil.com
Spectrophotometer Labeled Isotopes And Mass Spectrometry The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. in 1913, thomson. Isotopes And Mass Spectrometry.
From alevelchemistry.co.uk
Isotopes and Mass Spectrometry A Level Chemistry Revision Notes Isotopes And Mass Spectrometry we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. First, there is an ionization source, where the molecule is given a positive electrical charge, either by. Isotopes And Mass Spectrometry.
From chem.libretexts.org
13.3 Isotopes in Mass Spectrometry Chemistry LibreTexts Isotopes And Mass Spectrometry The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. the basics of a mass spectrometry experiment. we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. isotopes are nuclides that differ from each other by their mass number rather than. Isotopes And Mass Spectrometry.
From serc.carleton.edu
Gas Source Mass Spectrometry Stable Isotope Geochemistry Isotopes And Mass Spectrometry When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. in 1913, thomson mentioned a weak line at mass 22 accompanying. Isotopes And Mass Spectrometry.
From www.researchgate.net
Schematic of a continuous flow isotoperatio mass spectrometer Isotopes And Mass Spectrometry There are many different types of ms instruments, but they all have the same three essential components. First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding a proton. mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in.. Isotopes And Mass Spectrometry.
From www.studocu.com
AP chem Isotopes and Mass Spectrometry A PSI AP Chemistry Activity Isotopes And Mass Spectrometry isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. we’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. There are many different types of ms instruments, but they all have the same three essential components. the basics of. Isotopes And Mass Spectrometry.
From wisc.pb.unizin.org
Isotopes, Atomic Mass, and Mass Spectrometry (M2Q3) UWMadison Isotopes And Mass Spectrometry When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. There are many different types of ms instruments, but they all have the same. Isotopes And Mass Spectrometry.
From www.geology.pitt.edu
Isotope Ratio Mass Spectrometers Department of Geology and Isotopes And Mass Spectrometry the basics of a mass spectrometry experiment. When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding. Isotopes And Mass Spectrometry.
From www.youtube.com
Mass Spectrometry (Part 2) Spectrum, Molecular ion peak & Base Peak Isotopes And Mass Spectrometry mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. There are many different types of ms instruments, but they all have the same three essential components. isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. The existence of isotopes was first. Isotopes And Mass Spectrometry.
From www.thermofisher.com
Isotope Ratio Mass Spectrometry (IRMS) Thermo Fisher Scientific US Isotopes And Mass Spectrometry isotopes are nuclides that differ from each other by their mass number rather than their atomic number (fig. mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. There are many different types of ms instruments, but they all have the same three essential components. The existence of isotopes was first. Isotopes And Mass Spectrometry.
From chem.libretexts.org
13.3 Isotopes in Mass Spectrometry Chemistry LibreTexts Isotopes And Mass Spectrometry in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. the basics of a mass spectrometry experiment. we’ll examine how we use mass spectrometry to precisely measure the. Isotopes And Mass Spectrometry.
From www.geology.pitt.edu
Isotope Ratio Mass Spectrometers Department of Geology and Isotopes And Mass Spectrometry mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. the basics of a mass spectrometry experiment. The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. First, there is an ionization source, where the molecule is given a positive electrical charge, either by. Isotopes And Mass Spectrometry.
From duw.unibas.ch
Isotope Ratio Mass Spectrometry Departement Umweltwissenschaften Isotopes And Mass Spectrometry When interpreting mass spectra it is important to remember that the relative atomic mass or atomic weight of an element is a weighted average of the naturally occurring isotopes. mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. The existence of isotopes was first observed by aston using a mass spectrometer. Isotopes And Mass Spectrometry.
From www.thermofisher.com
Gas Isotope Ratio Mass Spectrometry (IRMS) Thermo Fisher Scientific US Isotopes And Mass Spectrometry First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron or by adding a proton. in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. isotopes are nuclides that differ from each other by their mass. Isotopes And Mass Spectrometry.
From duw.unibas.ch
Isotope Ratio Mass Spectrometry Departement Umweltwissenschaften Isotopes And Mass Spectrometry There are many different types of ms instruments, but they all have the same three essential components. The existence of isotopes was first observed by aston using a mass spectrometer to study neon ions. mass spectrometry, analytic technique by which chemical substances are identified by the sorting of gaseous ions in. isotopes are nuclides that differ from each. Isotopes And Mass Spectrometry.
From www.slideserve.com
PPT Isotopes & Mass Spectrometry PowerPoint Presentation, free Isotopes And Mass Spectrometry in 1913, thomson mentioned a weak line at mass 22 accompanying the expected one at mass 20 when he analyzed the mass. There are many different types of ms instruments, but they all have the same three essential components. First, there is an ionization source, where the molecule is given a positive electrical charge, either by removing an electron. Isotopes And Mass Spectrometry.