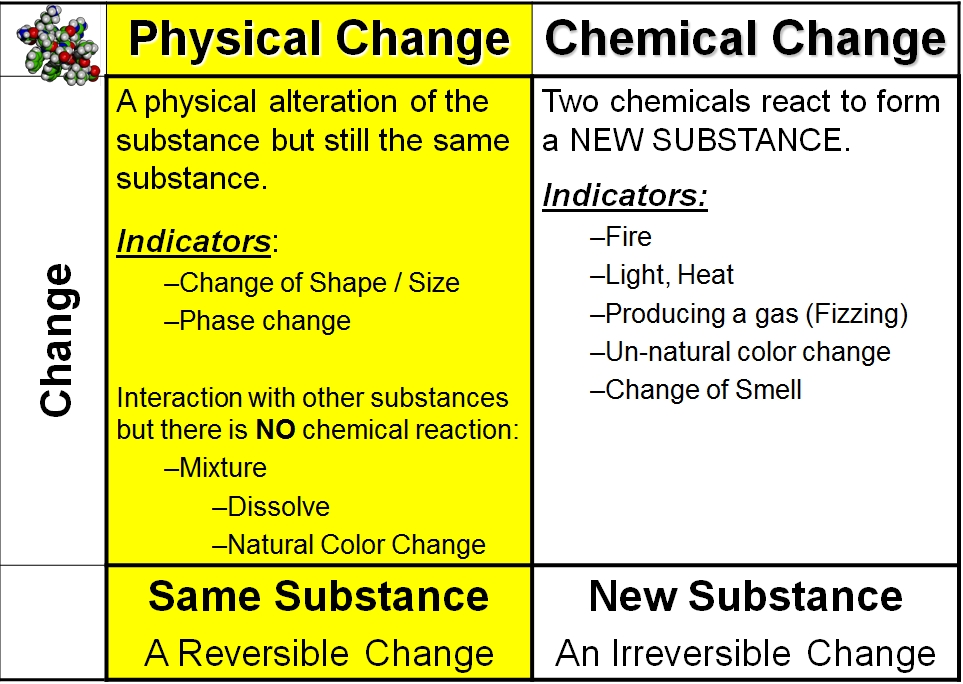
Is Phase Change Physical Or Chemical . Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase. In a chemical reaction, there is a change in the composition of the substances in question; Usually the change occurs when adding or removing heat at a particular. In a physical change there is a difference in the appearance, smell, or simple display of a. Similarly, when a material changes phase, it only changes physically; A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. A phase change is a physical process in which a substance goes from one phase to another. Think about ice melting into water, and then water being heated up and. The substance is still the same.
from vhmsscience.weebly.com
Changes of state are examples of phase. Think about ice melting into water, and then water being heated up and. The substance is still the same. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Usually the change occurs when adding or removing heat at a particular. Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. A phase change is a physical process in which a substance goes from one phase to another. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. In a chemical reaction, there is a change in the composition of the substances in question; Similarly, when a material changes phase, it only changes physically;
Chemical & Physical Changes VISTA HEIGHTS 8TH GRADE SCIENCE
Is Phase Change Physical Or Chemical The substance is still the same. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. In a physical change there is a difference in the appearance, smell, or simple display of a. Similarly, when a material changes phase, it only changes physically; Changes of state are examples of phase. In a chemical reaction, there is a change in the composition of the substances in question; A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. The substance is still the same. Think about ice melting into water, and then water being heated up and. A phase change is a physical process in which a substance goes from one phase to another. Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Usually the change occurs when adding or removing heat at a particular.
From materialdbhutchins.z21.web.core.windows.net
Heat During Phase Change Formula Is Phase Change Physical Or Chemical Similarly, when a material changes phase, it only changes physically; A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. In a chemical reaction, there is a change in the composition of the substances in question; Think about ice melting into water, and then water being heated up and. Changes of. Is Phase Change Physical Or Chemical.
From unistudium.unipg.it
Phase Diagrams Is Phase Change Physical Or Chemical Changes of state are examples of phase. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Similarly, when a material changes phase, it only changes physically; The substance is still the same. Usually the change occurs when adding or removing heat at a particular. Fusion, vaporization, and sublimation are endothermic. Is Phase Change Physical Or Chemical.
From sciencenotes.org
Examples of Chemical Change and How to Recognize It Is Phase Change Physical Or Chemical Changes of state are examples of phase. A phase change is a physical process in which a substance goes from one phase to another. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Think about ice melting into water, and then water being heated up and. Similarly, when a material. Is Phase Change Physical Or Chemical.
From sciencenotes.org
Chemical and Physical Changes of Matter Is Phase Change Physical Or Chemical Similarly, when a material changes phase, it only changes physically; Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Think about ice melting into water, and then water being heated up and. In a chemical reaction, there is a change in the composition of the substances in question;. Is Phase Change Physical Or Chemical.
From www.slideserve.com
PPT Physical & chemical changes PowerPoint Presentation, free download ID2431633 Is Phase Change Physical Or Chemical Usually the change occurs when adding or removing heat at a particular. Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Changes of state are examples of phase. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. In. Is Phase Change Physical Or Chemical.
From www.alamy.com
Phase changes of matter Stock Photo 84972731 Alamy Is Phase Change Physical Or Chemical Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Usually the change occurs when adding or removing heat at a particular. A phase change is a physical process in which a substance goes from one phase to another. Changes of state are examples of phase. Similarly, when a material changes phase, it only changes. Is Phase Change Physical Or Chemical.
From www.sliderbase.com
Phase Diagrams Presentation Chemistry Is Phase Change Physical Or Chemical A phase change is a physical process in which a substance goes from one phase to another. Similarly, when a material changes phase, it only changes physically; Think about ice melting into water, and then water being heated up and. In a chemical reaction, there is a change in the composition of the substances in question; The substance is still. Is Phase Change Physical Or Chemical.
From general.chemistrysteps.com
Heat and Phase Change Diagrams Chemistry Steps Is Phase Change Physical Or Chemical The substance is still the same. A phase change is a physical process in which a substance goes from one phase to another. Similarly, when a material changes phase, it only changes physically; In a physical change there is a difference in the appearance, smell, or simple display of a. Usually the change occurs when adding or removing heat at. Is Phase Change Physical Or Chemical.
From slidetodoc.com
Chemical and Physical Changes Physical Changes Physical Changes Is Phase Change Physical Or Chemical Think about ice melting into water, and then water being heated up and. In a physical change there is a difference in the appearance, smell, or simple display of a. Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. The substance is still the same. A phase change. Is Phase Change Physical Or Chemical.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Is Phase Change Physical Or Chemical Changes of state are examples of phase. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. In a physical change there is a difference in the appearance, smell, or simple display of a. The substance. Is Phase Change Physical Or Chemical.
From sciencenotes.org
Chemical and Physical Changes of Matter Is Phase Change Physical Or Chemical Changes of state are examples of phase. A phase change is a physical process in which a substance goes from one phase to another. Think about ice melting into water, and then water being heated up and. In a chemical reaction, there is a change in the composition of the substances in question; In a physical change there is a. Is Phase Change Physical Or Chemical.
From www.chemistrylearner.com
Physical Change Definition, Types, Examples, and Equations Is Phase Change Physical Or Chemical Think about ice melting into water, and then water being heated up and. In a physical change there is a difference in the appearance, smell, or simple display of a. Usually the change occurs when adding or removing heat at a particular. A phase change is a physical process in which a substance goes from one phase to another. Phase. Is Phase Change Physical Or Chemical.
From www.shmoop.com
Chemistry Phase Changes Shmoop Chemistry Is Phase Change Physical Or Chemical Similarly, when a material changes phase, it only changes physically; A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Usually the change occurs when adding or removing heat at a particular. A phase change is a physical process in which a substance goes from one phase to another. Phase changes. Is Phase Change Physical Or Chemical.
From www.slideserve.com
PPT Phases, Phase Changes, Chemical and Physical Changes PowerPoint Presentation ID2275834 Is Phase Change Physical Or Chemical In a chemical reaction, there is a change in the composition of the substances in question; Similarly, when a material changes phase, it only changes physically; Think about ice melting into water, and then water being heated up and. Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed.. Is Phase Change Physical Or Chemical.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Is Phase Change Physical Or Chemical A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Usually the change occurs when adding or removing heat at a particular. In a physical change there is a difference in the appearance, smell, or simple display of a. The substance is still the same. In a chemical reaction, there is. Is Phase Change Physical Or Chemical.
From www.pinterest.com.au
Changes in Matter 1 Matter science, Changes in matter, Chemical changes Is Phase Change Physical Or Chemical In a physical change there is a difference in the appearance, smell, or simple display of a. A phase change is a physical process in which a substance goes from one phase to another. Similarly, when a material changes phase, it only changes physically; Changes of state are examples of phase. The substance is still the same. Think about ice. Is Phase Change Physical Or Chemical.
From mobilesecret0.bitbucket.io
Properties Of Chemical Change Nzqa Physics Level 3 Formula Sheet Is Phase Change Physical Or Chemical Changes of state are examples of phase. Similarly, when a material changes phase, it only changes physically; A phase change is a physical process in which a substance goes from one phase to another. Usually the change occurs when adding or removing heat at a particular. A phase change or phase transition is a change between solid, liquid, gaseous, and. Is Phase Change Physical Or Chemical.
From www.online-sciences.com
What is the difference between the physical changes and the chemical changes? Science online Is Phase Change Physical Or Chemical Similarly, when a material changes phase, it only changes physically; Usually the change occurs when adding or removing heat at a particular. Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. The substance. Is Phase Change Physical Or Chemical.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Is Phase Change Physical Or Chemical Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. In a chemical reaction, there is a change in the composition of the substances in question; Usually the change occurs when adding or removing heat at a particular. Think about ice melting into water, and then water being heated up and. A phase change or. Is Phase Change Physical Or Chemical.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Reactions MCAT Content Is Phase Change Physical Or Chemical Similarly, when a material changes phase, it only changes physically; In a chemical reaction, there is a change in the composition of the substances in question; In a physical change there is a difference in the appearance, smell, or simple display of a. Think about ice melting into water, and then water being heated up and. Usually the change occurs. Is Phase Change Physical Or Chemical.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID2020516 Is Phase Change Physical Or Chemical A phase change is a physical process in which a substance goes from one phase to another. The substance is still the same. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. In a physical. Is Phase Change Physical Or Chemical.
From www.animalia-life.club
Chemical Change Vs Physical Change Is Phase Change Physical Or Chemical Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. Similarly, when a material changes phase, it only changes physically; Usually the change occurs when adding or removing heat at. Is Phase Change Physical Or Chemical.
From pediaa.com
Difference Between Physical and Chemical Change Definition, Examples Is Phase Change Physical Or Chemical Think about ice melting into water, and then water being heated up and. Similarly, when a material changes phase, it only changes physically; Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. In a physical change there is a difference in the appearance, smell, or simple display of. Is Phase Change Physical Or Chemical.
From www.slideserve.com
PPT Phase Changes & Phase Diagrams PowerPoint Presentation, free download ID5492277 Is Phase Change Physical Or Chemical Similarly, when a material changes phase, it only changes physically; In a physical change there is a difference in the appearance, smell, or simple display of a. The substance is still the same. Usually the change occurs when adding or removing heat at a particular. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes. Is Phase Change Physical Or Chemical.
From www.thoughtco.com
Examples of Physical Changes and Chemical Changes Is Phase Change Physical Or Chemical Think about ice melting into water, and then water being heated up and. A phase change is a physical process in which a substance goes from one phase to another. In a chemical reaction, there is a change in the composition of the substances in question; Usually the change occurs when adding or removing heat at a particular. The substance. Is Phase Change Physical Or Chemical.
From sciencenotes.org
Physical Change Examples Is Phase Change Physical Or Chemical A phase change is a physical process in which a substance goes from one phase to another. Similarly, when a material changes phase, it only changes physically; Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition. Is Phase Change Physical Or Chemical.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Is Phase Change Physical Or Chemical Usually the change occurs when adding or removing heat at a particular. In a chemical reaction, there is a change in the composition of the substances in question; Changes of state are examples of phase. A phase change is a physical process in which a substance goes from one phase to another. Similarly, when a material changes phase, it only. Is Phase Change Physical Or Chemical.
From vhmsscience.weebly.com
Chemical & Physical Changes VISTA HEIGHTS 8TH GRADE SCIENCE Is Phase Change Physical Or Chemical In a chemical reaction, there is a change in the composition of the substances in question; Think about ice melting into water, and then water being heated up and. Similarly, when a material changes phase, it only changes physically; Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. A phase change or phase transition. Is Phase Change Physical Or Chemical.
From www.slideserve.com
PPT Matter, Elements, Compounds and Mixtures PowerPoint Presentation ID3767196 Is Phase Change Physical Or Chemical Changes of state are examples of phase. Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Usually the change occurs when adding or removing heat at a particular. A phase change is a physical process in which a substance goes from one phase to another. A phase change. Is Phase Change Physical Or Chemical.
From shaunmwilliams.com
Chapter 1 Presentation Is Phase Change Physical Or Chemical Usually the change occurs when adding or removing heat at a particular. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. A phase change is a physical process in which a substance goes from one phase to another. The substance is still the same. Fusion, vaporization, and sublimation are endothermic. Is Phase Change Physical Or Chemical.
From slideplayer.com
Physical & Chemical Changes ppt download Is Phase Change Physical Or Chemical In a physical change there is a difference in the appearance, smell, or simple display of a. Usually the change occurs when adding or removing heat at a particular. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Think about ice melting into water, and then water being heated up and. A phase change. Is Phase Change Physical Or Chemical.
From www.slideserve.com
PPT Phases, Phase Changes, Chemical and Physical Changes PowerPoint Presentation ID1850148 Is Phase Change Physical Or Chemical Changes of state are examples of phase. Think about ice melting into water, and then water being heated up and. The substance is still the same. In a physical change there is a difference in the appearance, smell, or simple display of a. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states. Is Phase Change Physical Or Chemical.
From chemsimplified.com
Changes…Changes…Changes…Is it Physical or Chemical? ChemSimplified Is Phase Change Physical Or Chemical Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. In a chemical reaction, there is a change in the composition of the substances in question; Similarly, when a material changes phase, it only changes physically; A phase change is a physical process in which a substance goes from. Is Phase Change Physical Or Chemical.
From kentchemistry.com
Phase Changes Is Phase Change Physical Or Chemical In a physical change there is a difference in the appearance, smell, or simple display of a. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. Think about ice melting into water, and. Is Phase Change Physical Or Chemical.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID3746915 Is Phase Change Physical Or Chemical Changes of state are examples of phase. A phase change is a physical process in which a substance goes from one phase to another. The substance is still the same. In a chemical reaction, there is a change in the composition of the substances in question; In a physical change there is a difference in the appearance, smell, or simple. Is Phase Change Physical Or Chemical.