What Electrons Shared Between Atoms . Usually, sharing electrons gives each atom a full valence shell and. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. The electrons involved are in the outer shells of the atoms. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using. Chemists frequently use lewis diagrams. An atom that shares one or more of its. Two atoms form a bond to increase their stability,. When electrons are shared between two atoms, they make a bond called a covalent bond.
from owlcation.com
Chemists frequently use lewis diagrams. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using. Usually, sharing electrons gives each atom a full valence shell and. The electrons involved are in the outer shells of the atoms. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). An atom that shares one or more of its. When electrons are shared between two atoms, they make a bond called a covalent bond.
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind
What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. The electrons involved are in the outer shells of the atoms. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. Two atoms form a bond to increase their stability,. Let us illustrate a covalent bond by using. Chemists frequently use lewis diagrams. When electrons are shared between two atoms, they make a bond called a covalent bond. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). When electrons are shared between two atoms, they make a bond called a covalent bond. An atom that shares one or more of its. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing electrons gives each atom a full valence shell and.
From www.slideserve.com
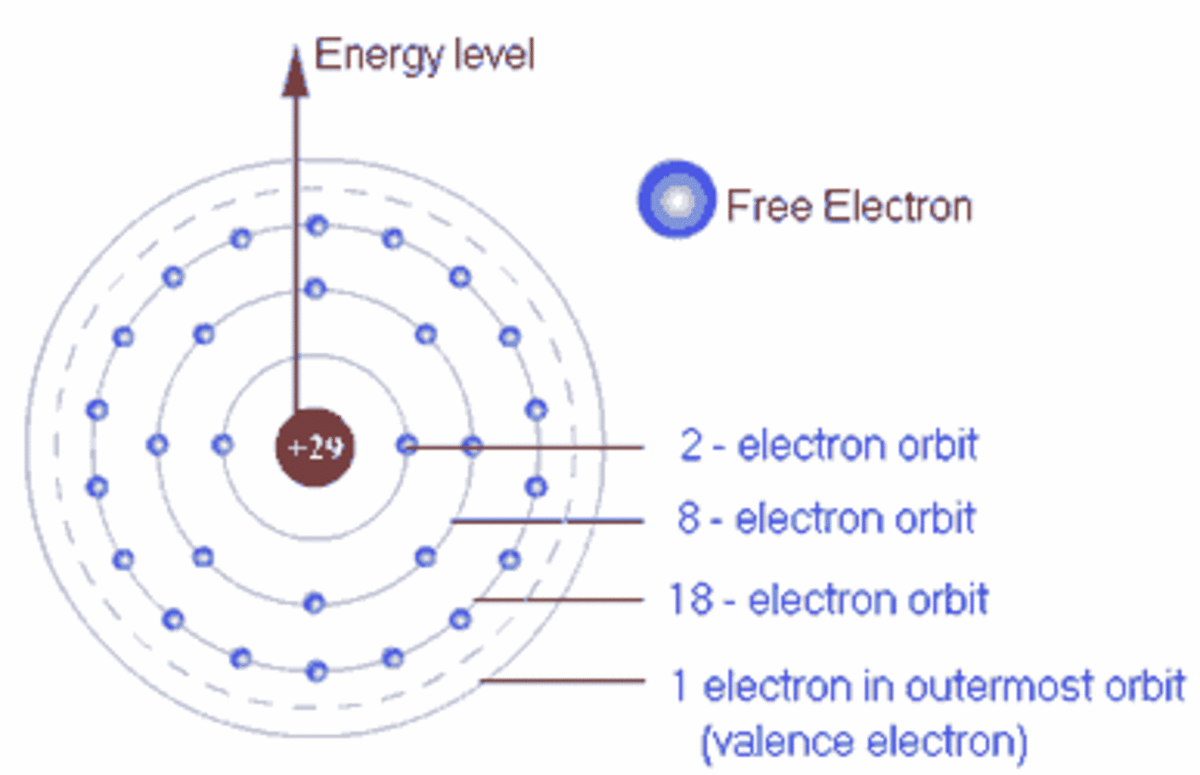
PPT Identify that some electrons in solids are shared between atoms What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. The electrons involved are in the outer shells of the atoms. Usually, sharing electrons gives each atom a full valence shell and. An atom that shares one or more of its. The total number of electrons around each individual atom consists of six nonbonding electrons. What Electrons Shared Between Atoms.
From learninglab.rmit.edu.au
Covalent bonds Learning Lab What Electrons Shared Between Atoms Let us illustrate a covalent bond by using. An atom that shares one or more of its. When electrons are shared between two atoms, they make a bond called a covalent bond. When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a full valence shell and. Chemists frequently. What Electrons Shared Between Atoms.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry What Electrons Shared Between Atoms Two atoms form a bond to increase their stability,. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. An atom that shares one or more of its. When electrons are shared between two atoms, they make a bond called a covalent bond. The total number of electrons around each individual atom. What Electrons Shared Between Atoms.
From www.expii.com
Electrons — Structure & Properties Expii What Electrons Shared Between Atoms The electrons involved are in the outer shells of the atoms. Usually, sharing electrons gives each atom a full valence shell and. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. An atom that shares one or more of its. Let us illustrate a covalent bond by using. The total. What Electrons Shared Between Atoms.
From masterconceptsinchemistry.com
How hydrogen atoms share valence electrons to form covalent bond and What Electrons Shared Between Atoms The electrons involved are in the outer shells of the atoms. An atom that shares one or more of its. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Chemists frequently use lewis diagrams. Let us illustrate a covalent bond by using. The total number of electrons around each individual. What Electrons Shared Between Atoms.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation What Electrons Shared Between Atoms Two atoms form a bond to increase their stability,. Let us illustrate a covalent bond by using. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. When electrons are shared between two atoms, they make a bond called a covalent bond. When electrons are shared between two atoms, they make a. What Electrons Shared Between Atoms.
From www.blog44.ca
Science Unit 3 Chemistry From the Mind of Isabelle What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Two atoms form a bond to increase their stability,. Let us illustrate a covalent bond by using. The electrons involved are in the outer shells of. What Electrons Shared Between Atoms.
From sansona.github.io
Attractive Forces and Bonds What Electrons Shared Between Atoms According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. Chemists frequently use lewis diagrams. The electrons involved are in the outer shells of the atoms. Usually, sharing electrons gives each atom a full valence shell and. When electrons are shared between two atoms, they make a bond called a covalent bond.. What Electrons Shared Between Atoms.
From oerpub.github.io
The top panel in this figure shows two hydrogen atoms sharing two What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). Two atoms form. What Electrons Shared Between Atoms.
From www.pinterest.com
What Happens When Atoms Bond infographic diagram showing how electrons What Electrons Shared Between Atoms Usually, sharing electrons gives each atom a full valence shell and. The electrons involved are in the outer shells of the atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. An atom that shares one or more of its. Two atoms form a bond to increase their stability,. The total number of electrons. What Electrons Shared Between Atoms.
From www.slideserve.com
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation ID5979513 What Electrons Shared Between Atoms Usually, sharing electrons gives each atom a full valence shell and. When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Two atoms form a bond to increase their stability,. An atom that shares one or. What Electrons Shared Between Atoms.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind What Electrons Shared Between Atoms Two atoms form a bond to increase their stability,. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using. When electrons are shared between two atoms, they make. What Electrons Shared Between Atoms.
From www.nagwa.com
Question Video Recalling the Number of Electrons in a Double Covalent What Electrons Shared Between Atoms Usually, sharing electrons gives each atom a full valence shell and. When electrons are shared between two atoms, they make a bond called a covalent bond. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). Chemists frequently use lewis diagrams. The electrons involved are in the outer shells of the. What Electrons Shared Between Atoms.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). An atom that shares one or more of its. Let us illustrate a covalent bond by using. When electrons are shared between two atoms, they make. What Electrons Shared Between Atoms.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent What Electrons Shared Between Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Let us illustrate a covalent bond by using. Two atoms form a bond to increase their stability,. When electrons are shared between two atoms, they make a bond called a covalent bond. The total number of electrons around each individual atom. What Electrons Shared Between Atoms.
From www.alamy.com
String theory. From molecule, and atoms, to electrons, protons What Electrons Shared Between Atoms The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using. A covalent bond is a chemical bond between two atoms where they share one or more pairs of. What Electrons Shared Between Atoms.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. When electrons are shared between two atoms, they make a bond called a covalent bond. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. Let us illustrate a covalent bond by using. Usually, sharing electrons gives. What Electrons Shared Between Atoms.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a full valence shell and. Chemists frequently use lewis diagrams. An atom that shares one or more of its. Two atoms form a bond to increase their stability,. A covalent bond is a chemical bond between two atoms where. What Electrons Shared Between Atoms.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Electrons Shared Between Atoms An atom that shares one or more of its. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. The electrons involved are in the outer shells of the atoms. Let us illustrate a covalent bond by using. The total number of electrons around each individual atom consists of six nonbonding electrons. What Electrons Shared Between Atoms.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Electrons Shared Between Atoms The electrons involved are in the outer shells of the atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a full valence shell and. Let us illustrate a covalent bond by using. Chemists frequently use lewis diagrams. A covalent bond is a chemical bond between two atoms. What Electrons Shared Between Atoms.
From www.meritnation.com
What is the electronic configuriaotn of sodium atom an oxygen atom What Electrons Shared Between Atoms According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. An atom that shares one or more of its. Two atoms form a bond to increase their stability,. Chemists frequently use lewis diagrams. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons.. What Electrons Shared Between Atoms.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID1698471 What Electrons Shared Between Atoms Two atoms form a bond to increase their stability,. When electrons are shared between two atoms, they make a bond called a covalent bond. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond. What Electrons Shared Between Atoms.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.44 Know that a Covalent Bond is Formed Between What Electrons Shared Between Atoms The electrons involved are in the outer shells of the atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a full valence shell and. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. An atom that shares one. What Electrons Shared Between Atoms.
From santiagomeowmeyer.blogspot.com
Why Are Only Valence Electrons Involved in Bonding What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. Chemists frequently use lewis diagrams. An atom that shares one or more of its. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing electrons gives each atom a full valence shell and. Two. What Electrons Shared Between Atoms.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. An atom that shares one or more of its. When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing. What Electrons Shared Between Atoms.
From byjus.com
The total number of electrons shared between the atoms of a hydrogen What Electrons Shared Between Atoms The electrons involved are in the outer shells of the atoms. Chemists frequently use lewis diagrams. Two atoms form a bond to increase their stability,. When electrons are shared between two atoms, they make a bond called a covalent bond. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). According. What Electrons Shared Between Atoms.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Electrons Shared Between Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. The electrons involved are in the outer shells of the atoms. Chemists frequently use lewis diagrams. When electrons are shared between two atoms, they make a bond called a covalent bond. When electrons are shared between two atoms, they make a. What Electrons Shared Between Atoms.
From www.vrogue.co
Solved Determine How Many Electrons Are Being Transfe vrogue.co What Electrons Shared Between Atoms Let us illustrate a covalent bond by using. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. Two atoms form a bond to increase their stability,. Usually, sharing electrons gives each atom. What Electrons Shared Between Atoms.
From kunduz.com
[ANSWERED] When electrons are shared between atoms in a union what bond What Electrons Shared Between Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Two atoms form a bond to increase their stability,. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its. What Electrons Shared Between Atoms.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID What Electrons Shared Between Atoms The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). An atom that shares one or more of its. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. Two atoms form a bond to increase their stability,. A covalent bond is a. What Electrons Shared Between Atoms.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind What Electrons Shared Between Atoms An atom that shares one or more of its. When electrons are shared between two atoms, they make a bond called a covalent bond. The electrons involved are in the outer shells of the atoms. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). Let us illustrate a covalent bond. What Electrons Shared Between Atoms.
From www.teachoo.com
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds What Electrons Shared Between Atoms Chemists frequently use lewis diagrams. When electrons are shared between two atoms, they make a bond called a covalent bond. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. An atom that. What Electrons Shared Between Atoms.
From www.coursehero.com
[Solved] 29. State the number of electrons shared between the carbon What Electrons Shared Between Atoms According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. Let us illustrate a covalent bond by using. When electrons are shared between two atoms, they make a bond called a covalent bond. An atom that shares one or more of its. Usually, sharing electrons gives each atom a full valence shell. What Electrons Shared Between Atoms.
From www.numerade.com
SOLVED Text shared between two atoms? 17. In which of the structures What Electrons Shared Between Atoms Chemists frequently use lewis diagrams. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. When electrons are shared between two atoms, they make a bond called a covalent bond. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). According to. What Electrons Shared Between Atoms.
From resultshc.blogspot.com
Nonpolar Molecules Are The Result Of Unequal Electron Pair Sharing What Electrons Shared Between Atoms An atom that shares one or more of its. Let us illustrate a covalent bond by using. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding). According to langmuir, covalence is the number of pairs of electrons shared between an atom and its neighbor. When electrons are shared between two. What Electrons Shared Between Atoms.