Medical Device Packing List . Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. Usa regulations for medical device packaging. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. This type of packaging permits easier administration by means of devices such as. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices.
from betop20percent.com
Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Usa regulations for medical device packaging. This type of packaging permits easier administration by means of devices such as.
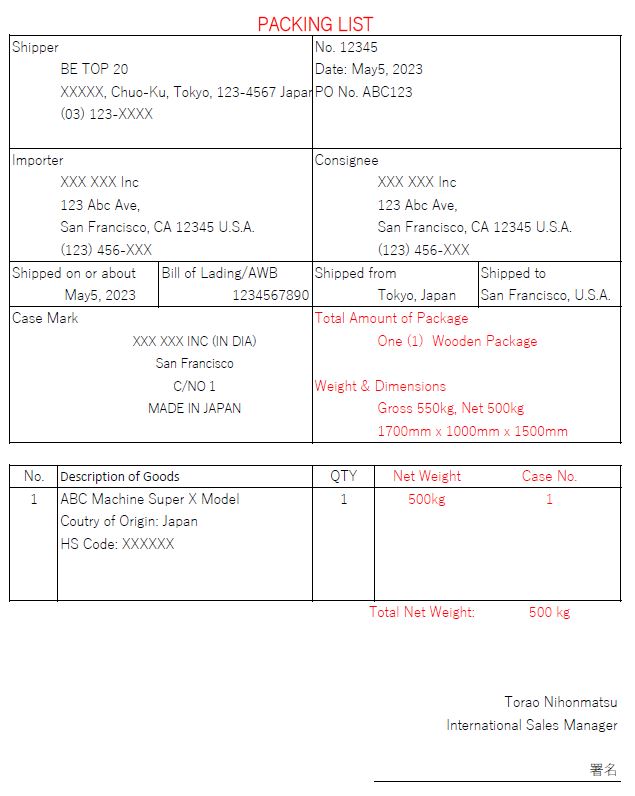
パッキングリストとは?輸出入通関で使用するパッキングリストの作り方。 びーとっぷ20
Medical Device Packing List Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Usa regulations for medical device packaging. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. This type of packaging permits easier administration by means of devices such as. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely.
From mavink.com
Modelo De Packing List Medical Device Packing List This type of packaging permits easier administration by means of devices such as. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. Fda is issuing this guidance to announce that. Medical Device Packing List.
From daydesigner.com
Packing Checklist for Vacation Printable Day Designer Medical Device Packing List Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. Usa regulations for medical device packaging. To help you identify the laws you need to comply with, here is a list. Medical Device Packing List.
From dextre.app
パッキングリストとは?フォーマットやコマーシャルインボイスとの違い【貿易書類作成のシステム化】 DEXTRE(デクスター) Medical Device Packing List Usa regulations for medical device packaging. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device packaging must comply with the quality system regulation standards. Medical Device Packing List.
From www.ossid.com
Medical Device Packaging Form Fill Seal Packing Equipment Medical Medical Device Packing List This type of packaging permits easier administration by means of devices such as. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. Indicates a medical device that should not be used. Medical Device Packing List.
From www.alibaba.com
Single Use Sterile Medical Device Packaging Bags Buy Sterile Bags Medical Device Packing List Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. Usa regulations for medical device packaging. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. To help you identify the laws you need to comply with, here is a list. Medical Device Packing List.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Medical Device Packing List Usa regulations for medical device packaging. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. This type of packaging permits easier administration by means of devices such as. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Fda is. Medical Device Packing List.
From www.nsmedicaldevices.com
Pentamed® Specialty Rigid Films NS Medical Devices Medical Device Packing List To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. Usa regulations for medical device packaging. Labeling and packaging is key to ensure that a medical device. Medical Device Packing List.
From www.slideshare.net
Symbols Commonly Used in Medical Device Packaging and Labeling Medical Device Packing List Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. This type of packaging permits easier administration by means of devices such as. Usa regulations for medical device packaging. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used. Medical Device Packing List.
From betop20percent.com
パッキングリストとは?輸出入通関で使用するパッキングリストの作り方。 びーとっぷ20 Medical Device Packing List To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Indicates a medical device that should not be used if the package has been damaged or opened and. Medical Device Packing List.
From passionplanner.com
Packing List PDF Free Printable Passion Planner Medical Device Packing List Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Indicates. Medical Device Packing List.
From www.sunplan.co.jp
【貿易事務】パッキングリストを間違えない!作成注意点と対応ソフト|サンプランソフト Medical Device Packing List Usa regulations for medical device packaging. This type of packaging permits easier administration by means of devices such as. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Indicates a medical device that should not be used if the package has been damaged or opened and that the. Medical Device Packing List.
From www.wanjiamedical.com
PACKING LIST Huai'an Wanjia Medical Device Co., Ltd. Medical Device Packing List Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. This type of packaging permits easier administration by means of devices such as. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. Labeling and packaging is key to ensure that a. Medical Device Packing List.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Packing List This type of packaging permits easier administration by means of devices such as. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Indicates a medical device. Medical Device Packing List.
From www.packcon.org
Steps to Designing a Sterile Medical Device Package Medical Device Packing List Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. This type of packaging permits easier administration by means of devices such as. Fda is issuing this guidance to announce that both. Medical Device Packing List.
From influencepackaging.com
Custom Packaging & Medical Device Packaging Influence Packaging Medical Device Packing List Usa regulations for medical device packaging. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Labeling and packaging is key to ensure that a medical device or. Medical Device Packing List.
From officetemplatesonline.com
Download Sample Packing List Template for MS Excel Medical Device Packing List To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Fda is issuing this guidance to announce that both the list of devices subject to medical device. Medical Device Packing List.
From mungfali.com
Medical Device Labeling Symbols Medical Device Packing List This type of packaging permits easier administration by means of devices such as. Usa regulations for medical device packaging. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. To help you identify the laws you need to comply with, here is a list of packaging regulations for. Medical Device Packing List.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Medical Device Packing List Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. This type of packaging permits easier administration by means of devices such as. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Usa regulations for medical. Medical Device Packing List.
From rs-ness.com
Medical Device Packaging Validation Guideline RS NESS Medical Device Packing List To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Usa. Medical Device Packing List.
From www.dreamstime.com
Medical Device Packaging Symbol Stock Illustrations 338 Medical Medical Device Packing List Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Usa regulations for medical device packaging. This type of packaging permits easier administration by means of devices. Medical Device Packing List.
From www.hamilton-medical.com
Changes to labels on packaging Hamilton Medical Medical Device Packing List Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. This type of packaging permits easier administration by means of devices such as. Usa regulations for medical device packaging. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements,. Medical Device Packing List.
From www.rockwellautomation.com
Medical Device Manufacturing Automation and Control Systems Rockwell Medical Device Packing List This type of packaging permits easier administration by means of devices such as. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Usa regulations for medical. Medical Device Packing List.
From www.kmedhealth.com
Medical Device Label SymbolsI In Packaging Medical Device Packing List This type of packaging permits easier administration by means of devices such as. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Fda is issuing. Medical Device Packing List.
From help.fishbowlonline.com
Fishbowl Drive Packing List Report Medical Device Packing List This type of packaging permits easier administration by means of devices such as. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Fda is issuing this. Medical Device Packing List.
From www.slideshare.net
Medical device Packaging Medical Device Packing List Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Usa regulations for medical device packaging. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Medical device packaging must comply with the quality system regulation standards. Medical Device Packing List.
From www.dreamstime.com
Full Set of International Medical Device Packaging Symbols with Medical Device Packing List Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. This type of packaging permits easier administration by means of devices such as. Usa regulations for medical device packaging. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. To help. Medical Device Packing List.
From www.dreamstime.com
Medical Packaging Symbols Stock Illustrations 374 Medical Packaging Medical Device Packing List Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can. Medical Device Packing List.
From onlinelibrary.wiley.com
Do Healthcare Professionals Comprehend Standardized Symbols Present on Medical Device Packing List Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. This type of packaging permits easier administration by means of devices such as. Usa regulations for medical device packaging. To help you identify the laws you need to comply with, here is a list of packaging regulations for. Medical Device Packing List.
From www.alamy.com
International Medical Packaging Symbols Stock Photo 40190601 Alamy Medical Device Packing List Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. This type of packaging permits easier administration by means of devices such as. To help you identify the laws you need. Medical Device Packing List.
From gracehealmedic.com
Medical Device Contract Packing GRACEHEAL MEDIC SDN. BHD. Medical Device Packing List Usa regulations for medical device packaging. Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. This type of packaging permits easier administration by means of devices such as. Indicates a medical device that should not be used if the package has been damaged or opened and that the. Medical Device Packing List.
From 99designs.com
Design a new package for a medical device company. Product packaging Medical Device Packing List Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which. Medical Device Packing List.
From www.sigma-medical.com.tw
ProductsMedical Sterilization Packaging Medical Device Packing List Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. This type of packaging permits easier administration by means of devices such as. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Labeling and packaging is. Medical Device Packing List.
From butsuryu-techo.com
INVOICE、PACKING LISTの作り方【書類サンプルあり】 物流手帖 Medical Device Packing List Labeling and packaging is key to ensure that a medical device or in vitro diagnostic device can be used effectively and safely. Usa regulations for medical device packaging. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. Indicates a medical device that should not be used if the package has been damaged. Medical Device Packing List.
From stock.adobe.com
Full set of medical device packaging symbols with warning information Medical Device Packing List Usa regulations for medical device packaging. To help you identify the laws you need to comply with, here is a list of packaging regulations for medical devices. Medical device packaging must comply with the quality system regulation standards (qsr 21 cfr 820), which outlines. This type of packaging permits easier administration by means of devices such as. Fda is issuing. Medical Device Packing List.
From hunade.com
貿易書類・パッキングリスト フォーマットも掲載! 【HUNADE】輸出入ガイド・国際物流・貿易業務代行 Medical Device Packing List Fda is issuing this guidance to announce that both the list of devices subject to medical device tracking requirements, and the. Usa regulations for medical device packaging. Indicates a medical device that should not be used if the package has been damaged or opened and that the user should. Labeling and packaging is key to ensure that a medical device. Medical Device Packing List.