What Is A Standard Reduction Potential . The standard reduction potential is +.34 volts. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. We know in a redox reaction something is reduced and something. Tabulated values used to calculate. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. 1 m for solutes) usually at 298.15 k; Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. This is equal to +.34 volts.
from www.slideserve.com
Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Tabulated values used to calculate. This is equal to +.34 volts. The standard reduction potential is +.34 volts. We know in a redox reaction something is reduced and something. 1 m for solutes) usually at 298.15 k; The value of the reduction under standard conditions (1 bar or 1 atm for gases; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced.
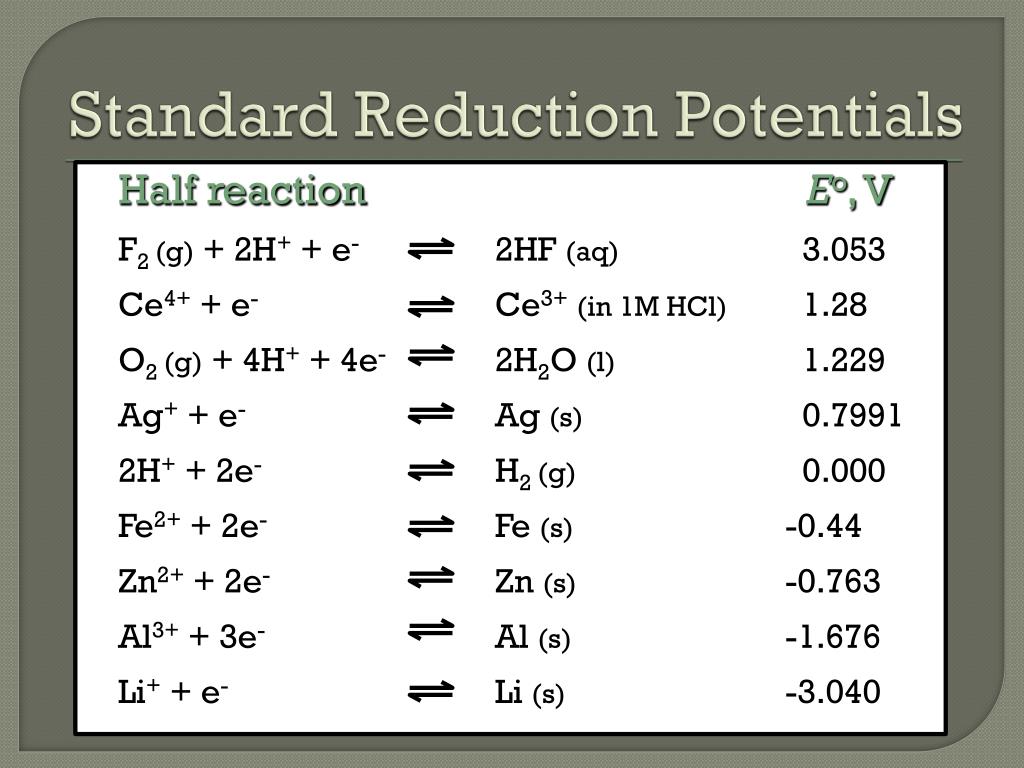
PPT Electrochemistry PowerPoint Presentation, free download ID2281515
What Is A Standard Reduction Potential We know in a redox reaction something is reduced and something. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. This is equal to +.34 volts. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. Tabulated values used to calculate. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. 1 m for solutes) usually at 298.15 k; Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. We know in a redox reaction something is reduced and something. The standard reduction potential is +.34 volts.
From pveducation.org
Standard Potential PVEducation What Is A Standard Reduction Potential The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. We. What Is A Standard Reduction Potential.
From www.chegg.com
Solved What is the standard reduction potential (x) for the What Is A Standard Reduction Potential Tabulated values used to calculate. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. What Is A Standard Reduction Potential.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint What Is A Standard Reduction Potential Tabulated values used to calculate. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The standard reduction potential is +.34 volts. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. The value of the reduction under standard. What Is A Standard Reduction Potential.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 What Is A Standard Reduction Potential We know in a redox reaction something is reduced and something. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. The standard reduction potential is +.34 volts. The value of. What Is A Standard Reduction Potential.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II What Is A Standard Reduction Potential Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Tabulated values used to calculate. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons. What Is A Standard Reduction Potential.
From ar.inspiredpencil.com
Standard Reduction Potential Table What Is A Standard Reduction Potential The standard reduction potential is +.34 volts. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Tabulated values used to calculate. The standard reduction potential, also known as e°red, is a measure of the. What Is A Standard Reduction Potential.
From www.slideserve.com
PPT Redox Reactions and Electrochemistry PowerPoint Presentation What Is A Standard Reduction Potential The standard reduction potential is +.34 volts. We know in a redox reaction something is reduced and something. Tabulated values used to calculate. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The. What Is A Standard Reduction Potential.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free What Is A Standard Reduction Potential The standard reduction potential is +.34 volts. This is equal to +.34 volts. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Tabulated values used to calculate. We know in a redox reaction something is reduced and something. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the. What Is A Standard Reduction Potential.
From www.nagwa.com
Question Video Calculating the Standard Reduction Potential of an What Is A Standard Reduction Potential The standard reduction potential is +.34 volts. Tabulated values used to calculate. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. This is equal to +.34 volts. 1 m for solutes) usually at 298.15 k; We know in a redox reaction something is reduced and something. Assigning the. What Is A Standard Reduction Potential.
From www.slideserve.com
PPT The Oxidation States of Vanadium PowerPoint Presentation ID4176570 What Is A Standard Reduction Potential The standard reduction potential is +.34 volts. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. 1 m for solutes) usually at 298.15 k; Assigning the potential of the. What Is A Standard Reduction Potential.
From ar.inspiredpencil.com
Standard Reduction Potential Table What Is A Standard Reduction Potential Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. 1 m for solutes) usually at 298.15 k; The standard reduction potential is +.34 volts. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. This is equal to +.34 volts.. What Is A Standard Reduction Potential.
From rayb78.github.io
Standard Reduction Potentials Chart What Is A Standard Reduction Potential This is equal to +.34 volts. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. The. What Is A Standard Reduction Potential.
From ch302.cm.utexas.edu
stdpotsshortlist.png What Is A Standard Reduction Potential Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. This is equal to +.34 volts. The standard reduction potential is +.34 volts. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. We know in a redox reaction something is reduced and something. The. What Is A Standard Reduction Potential.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is A Standard Reduction Potential This is equal to +.34 volts. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. The standard reduction potential is the tendency for a chemical species to be. What Is A Standard Reduction Potential.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free What Is A Standard Reduction Potential Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. This is equal to +.34 volts. The value of the reduction under standard conditions (1 bar or 1 atm. What Is A Standard Reduction Potential.
From www.pinterest.com
pe=log[e] measuved in volts.if pe is negative reduced, if pe is What Is A Standard Reduction Potential This is equal to +.34 volts. The standard reduction potential is +.34 volts. 1 m for solutes) usually at 298.15 k; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. We know in a redox reaction something is reduced and something. The standard reduction potential, also known as. What Is A Standard Reduction Potential.
From www.youtube.com
The standard reduction potential for `Cu^(2+)Cu` is `+0.34V What Is A Standard Reduction Potential The value of the reduction under standard conditions (1 bar or 1 atm for gases; Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. This is equal to +.34 volts. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Assigning. What Is A Standard Reduction Potential.
From vpia.org.vn
Standard Reduction Potentials made easy, reduction line What Is A Standard Reduction Potential The value of the reduction under standard conditions (1 bar or 1 atm for gases; Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential is +.34 volts. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and. What Is A Standard Reduction Potential.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 What Is A Standard Reduction Potential Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Tabulated values used to calculate. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. We know. What Is A Standard Reduction Potential.
From www.bartleby.com
Answered Selected Standard Reduction Potentials… bartleby What Is A Standard Reduction Potential Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. We know in a redox reaction something is reduced and something. This is equal to +.34 volts. The standard reduction potential. What Is A Standard Reduction Potential.
From www.chegg.com
Solved Use the standard reduction potentials located in the What Is A Standard Reduction Potential Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. This is equal to +.34 volts. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. 1. What Is A Standard Reduction Potential.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry What Is A Standard Reduction Potential 1 m for solutes) usually at 298.15 k; Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. Tabulated values used to calculate. The value of the reduction under standard. What Is A Standard Reduction Potential.
From www.pinterest.com
Standard Reduction Potential (E) when given two half reactions and What Is A Standard Reduction Potential Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. We know in a. What Is A Standard Reduction Potential.
From www.flinnsci.ca
Standard Reduction Potential Charts for Chemistry What Is A Standard Reduction Potential 1 m for solutes) usually at 298.15 k; We know in a redox reaction something is reduced and something. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Tabulated values used to calculate. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and. What Is A Standard Reduction Potential.
From www.youtube.com
Standard Reduction Potential YouTube What Is A Standard Reduction Potential The value of the reduction under standard conditions (1 bar or 1 atm for gases; Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. 1 m for solutes) usually at 298.15 k; Tabulated values used to calculate. This is equal to +.34 volts. The standard reduction potential, also known as e°red, is. What Is A Standard Reduction Potential.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download What Is A Standard Reduction Potential The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. The standard reduction potential is +.34 volts. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. This is equal to +.34 volts. The standard reduction potential is the. What Is A Standard Reduction Potential.
From www.youtube.com
Standard reduction potentials Redox reactions and electrochemistry What Is A Standard Reduction Potential The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. The standard reduction potential is +.34 volts. 1 m for solutes) usually at 298.15 k; We know in a redox reaction something is reduced and something. This is equal to +.34 volts. The value of the reduction under standard. What Is A Standard Reduction Potential.
From www.chegg.com
Solved TABLE 14.1 "Electron Tower" of Standard Reduction What Is A Standard Reduction Potential The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. Tabulated values used to calculate. We know in a redox reaction something is reduced and something. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced.. What Is A Standard Reduction Potential.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free What Is A Standard Reduction Potential We know in a redox reaction something is reduced and something. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. The standard reduction potential, also known as e°red, is a measure of the. What Is A Standard Reduction Potential.
From www.youtube.com
Difference between Oxidation potential and Reduction potential What Is A Standard Reduction Potential This is equal to +.34 volts. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. 1 m for solutes) usually at 298.15 k; Tabulated values used to calculate. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction.. What Is A Standard Reduction Potential.
From ar.inspiredpencil.com
Standard Reduction Potential Table What Is A Standard Reduction Potential The standard reduction potential is +.34 volts. 1 m for solutes) usually at 298.15 k; Tabulated values used to calculate. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The standard reduction potential is the tendency for. What Is A Standard Reduction Potential.
From www.researchgate.net
Redox potentials of some oxygen reduction and water oxidation reactions What Is A Standard Reduction Potential We know in a redox reaction something is reduced and something. Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. 1 m for solutes) usually at 298.15 k; Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The standard reduction potential is the. What Is A Standard Reduction Potential.
From www.researchgate.net
Gibbs free energy changes and standard reduction potentials at 25 • C What Is A Standard Reduction Potential Use standard reduction potentials to determine the better oxidizing or reducing agent from among several possible choices. We know in a redox reaction something is reduced and something. The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. Assigning the potential of the standard hydrogen electrode (she). What Is A Standard Reduction Potential.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download What Is A Standard Reduction Potential Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. The value of the reduction under standard conditions (1 bar or 1 atm for gases; We know in a redox reaction something is reduced and something. This is equal to +.34 volts. The standard reduction potential is the tendency for a chemical. What Is A Standard Reduction Potential.
From www.youtube.com
Standard Reduction Potentials of Half Reactions Electrochemistry What Is A Standard Reduction Potential 1 m for solutes) usually at 298.15 k; The standard reduction potential, also known as e°red, is a measure of the tendency of a species to gain electrons and be reduced. Tabulated values used to calculate. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard. This is equal. What Is A Standard Reduction Potential.