Magnesium + Copper (Ii) Nitrate Balanced Equation . If no reaction occurs, simply. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. Enter an equation of an ionic chemical equation and press the balance button. Let's balance this equation using the inspection method. The balanced equation will appear. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. The balanced equation will be calculated along with the. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. First, we set all coefficients to 1:
from www.slideserve.com
The balanced equation will appear. Let's balance this equation using the inspection method. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. First, we set all coefficients to 1: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. If no reaction occurs, simply. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of.
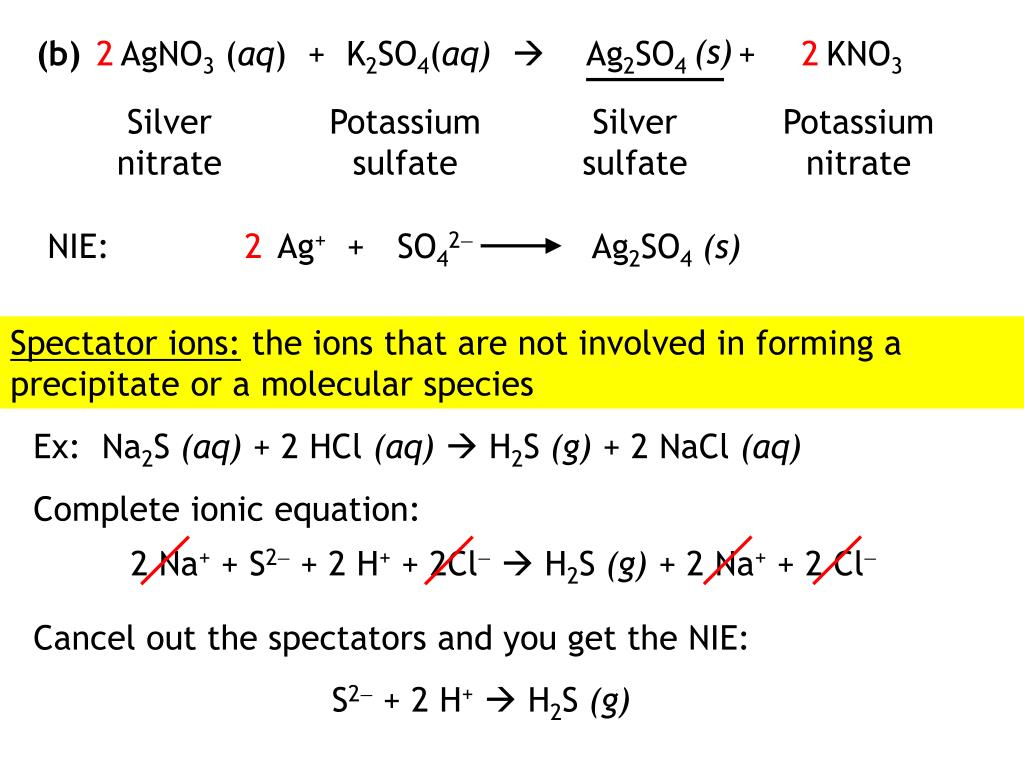
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry
Magnesium + Copper (Ii) Nitrate Balanced Equation Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. If no reaction occurs, simply. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. First, we set all coefficients to 1: Let's balance this equation using the inspection method. The balanced equation will be calculated along with the. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Enter an equation of an ionic chemical equation and press the balance button. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate.
From www.toppr.com
21. Express the following reaction in the form of a balanced chemical Magnesium + Copper (Ii) Nitrate Balanced Equation The balanced equation will be calculated along with the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. The coefficients indicate the number of each substance involved in the reaction and may be changed in. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Na2S + Zn(NO3)2 = NaNO3 + ZnS Magnesium + Copper (Ii) Nitrate Balanced Equation The balanced equation will be calculated along with the. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Enter an equation of an ionic chemical equation and press the balance button. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From slideplayer.com
Redox Reactions. Reduction Oxidation. ppt download Magnesium + Copper (Ii) Nitrate Balanced Equation There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation.. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.youtube.com
Writing Ionic Formulas Magnesium Nitrate YouTube Magnesium + Copper (Ii) Nitrate Balanced Equation If no reaction occurs, simply. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will appear. First, we set all coefficients to 1: The equation above indicates that one mole of. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVED balanced equation; Solutions of magnesium iodide and copper (II Magnesium + Copper (Ii) Nitrate Balanced Equation If no reaction occurs, simply. Let's balance this equation using the inspection method. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. To. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.youtube.com
How to Balance Mg(NO3)2 = MgO + NO2 + O2 (Magnesium nitrate) YouTube Magnesium + Copper (Ii) Nitrate Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. First, we set all coefficients to 1: Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. Enter. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(NO3)2 + (NH4)2S = CuS Magnesium + Copper (Ii) Nitrate Balanced Equation Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. If no reaction occurs,. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Magnesium + Copper (Ii) Nitrate Balanced Equation There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate.. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Magnesium + Copper (Ii) Nitrate Balanced Equation First, we set all coefficients to 1: 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. Let's balance this equation using the inspection method. Write a balanced equation for the decomposition of ammonium nitrate to. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.slideserve.com
PPT CHEMICAL REACTIONS PowerPoint Presentation, free download ID Magnesium + Copper (Ii) Nitrate Balanced Equation 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The balanced equation will be calculated along with the. If no reaction occurs, simply. Write a balanced equation for. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVEDWhich acid and base react to give an aqueous solution of Magnesium + Copper (Ii) Nitrate Balanced Equation The balanced equation will appear. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. If no reaction occurs, simply. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The coefficients indicate the number of each substance. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.chegg.com
Solved Write a balanced chemical equation for each of the Magnesium + Copper (Ii) Nitrate Balanced Equation Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu +. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation, free download ID Magnesium + Copper (Ii) Nitrate Balanced Equation There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. The balanced equation will appear. The coefficients indicate the number of each substance involved in the reaction and may be changed. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From testbook.com
Copper (II) Nitrate Formula Learn Structure, Properties, Uses Magnesium + Copper (Ii) Nitrate Balanced Equation The balanced equation will appear. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. First, we set all coefficients to 1: Write a balanced equation for the decomposition of ammonium. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.chegg.com
Solved Translate Each Word Equation into a Balanced Chemical Magnesium + Copper (Ii) Nitrate Balanced Equation First, we set all coefficients to 1: The balanced equation will appear. Enter an equation of an ionic chemical equation and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The coefficients indicate the number of each substance involved in the. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.chegg.com
Solved VI. Write formula equations and balance with Magnesium + Copper (Ii) Nitrate Balanced Equation The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. Enter an equation of an ionic chemical equation and press the balance button. Let's balance this equation using the inspection method. There are three main steps for writing the net ionic equation for mg + cu (no3)2 =. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVED 12 Write 3 balanced equation for the reaction of aqueous Magnesium + Copper (Ii) Nitrate Balanced Equation The balanced equation will appear. Enter an equation of an ionic chemical equation and press the balance button. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. Let's balance this equation using the inspection method. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button.. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced equation for Magnesium nitride + water Magnesium + Copper (Ii) Nitrate Balanced Equation First, we set all coefficients to 1: There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. If no reaction occurs, simply. Let's balance this equation using the inspection method. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVED Text Copper II nitrate and calcium hydroxide Balanced Equation Magnesium + Copper (Ii) Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. First, we set all coefficients to 1: The balanced equation will be calculated along with the. Write the balanced molecular chemical equation for. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.toppr.com
Write a balanced chemical equation with the help of chemical symbols Magnesium + Copper (Ii) Nitrate Balanced Equation If no reaction occurs, simply. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate.. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVED 1. When copper (II) chloride reacts with sodium nitrate, copper Magnesium + Copper (Ii) Nitrate Balanced Equation There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVED Part 1 [Copper metal to Copper (II) nitrate] 2pts. Balanced Magnesium + Copper (Ii) Nitrate Balanced Equation First, we set all coefficients to 1: The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Let's balance this equation using the inspection method. Write a balanced equation for the decomposition of ammonium nitrate to form. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Magnesium + Copper (Ii) Nitrate Balanced Equation Let's balance this equation using the inspection method. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The balanced equation will be calculated along. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Magnesium + Copper (Ii) Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. First, we set all coefficients to 1: Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen,. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.chegg.com
Solved When Copper (II) Chloride Reacts With Sodium Nitra... Magnesium + Copper (Ii) Nitrate Balanced Equation First, we set all coefficients to 1: There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. The balanced equation will appear. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. If no reaction occurs, simply. Write a balanced equation for. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.youtube.com
How to Write the Formula for Magnesium nitrate YouTube Magnesium + Copper (Ii) Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. If no reaction occurs, simply. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. The. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.youtube.com
IGCSE Chemistry lesson 34 part c Thermal of nitrates Magnesium + Copper (Ii) Nitrate Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. The balanced equation will. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVEDWhen the following molecular equation is balanced using the sina Magnesium + Copper (Ii) Nitrate Balanced Equation The balanced equation will be calculated along with the. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. First, we set all coefficients to 1: To balance a. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.meritnation.com
Write balanced chemical equations for the conversions A,B C A Copper Magnesium + Copper (Ii) Nitrate Balanced Equation First, we set all coefficients to 1: The balanced equation will be calculated along with the. Let's balance this equation using the inspection method. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. There are. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVED The video shows the addition of aqueous sodium carbonate to a Magnesium + Copper (Ii) Nitrate Balanced Equation The balanced equation will appear. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the equation. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. Enter an equation of an ionic chemical equation and press the balance. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From lillyfersmcknight.blogspot.com
Copper Silver Nitrate Yields Copper Ii Nitrate Silver Magnesium + Copper (Ii) Nitrate Balanced Equation Write the balanced molecular chemical equation for the reaction of solid magnesium with aqueous copper (ii) nitrate. Let's balance this equation using the inspection method. First, we set all coefficients to 1: The balanced equation will appear. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVED thermal of magnesium nitrate and magnesium hydroxide Magnesium + Copper (Ii) Nitrate Balanced Equation If no reaction occurs, simply. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will be calculated along with the. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. Let's balance this equation using the inspection method.. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.youtube.com
Mg(NO3)2=MgO +NO2+O2 Balanced EquationMagnesium Nitrate=Magnesium Magnesium + Copper (Ii) Nitrate Balanced Equation If no reaction occurs, simply. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. Enter an equation of an ionic chemical equation and press the balance button. The coefficients indicate the number of each substance involved in the reaction and may be changed in order to balance the. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVEDWe can tell from the formula of a salt how it can be produced Magnesium + Copper (Ii) Nitrate Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Let's balance this equation using the inspection method. The balanced equation will appear. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. There are three main steps for writing the net ionic equation for. Magnesium + Copper (Ii) Nitrate Balanced Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation showing the reaction between Magnesium + Copper (Ii) Nitrate Balanced Equation The balanced equation will be calculated along with the. If no reaction occurs, simply. There are three main steps for writing the net ionic equation for mg + cu (no3)2 = cu + mg (no3)2. 1 mg + 1 cu(no 3) 2 = 1 mg(no 3) 2 + 1 cu for each. To balance a chemical equation, enter an equation. Magnesium + Copper (Ii) Nitrate Balanced Equation.