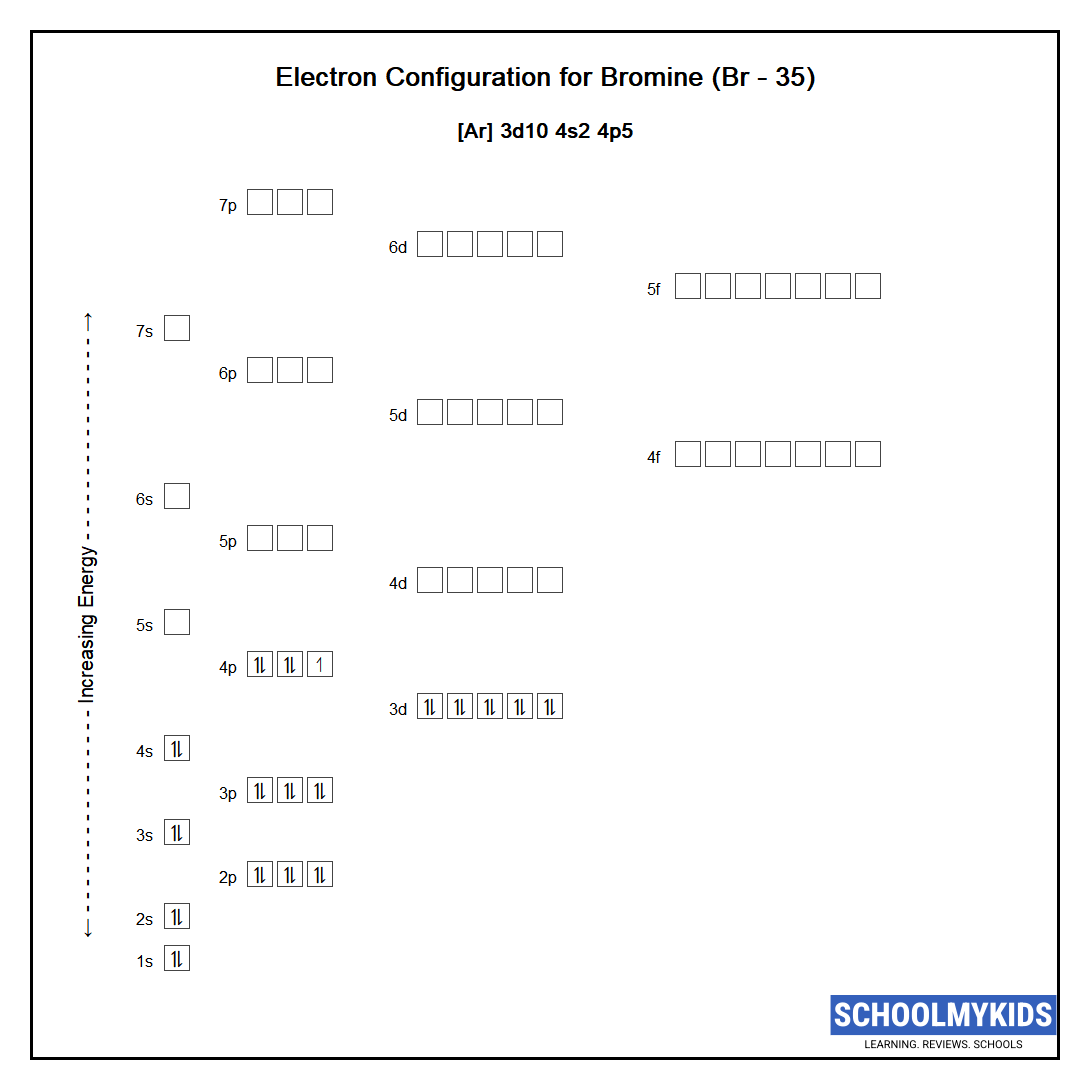
Bromine Atom Electron Configuration . Electron configuration of bromine is [ar] 3d10 4s2 4p5. Members of a group typically have similar properties and electron configurations in their outer shell. Most common application of bromine. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. This can be shortened to [ar]4s23d104p5. Electron configuration chart of all elements is mentioned in the table below. Bromine is intermediate in reactivity between chlorine. Br (bromine) is an element with position number. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Use a chart such as the one below. ← electronic configurations of elements. The shorthand electron configuration (or noble gas configuration) as well as. The atomic number of bromine is 35. Bromine (br) is a chemical element. A horizontal row in the periodic.
from www.schoolmykids.com
Use a chart such as the one below. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. The atomic number of bromine is 35. Most common application of bromine. Bromine (br) is a chemical element. Br (bromine) is an element with position number. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. Electron configuration of bromine is [ar] 3d10 4s2 4p5. A horizontal row in the periodic.
Bromine (Br) Element Information, Facts, Properties, Uses Periodic
Bromine Atom Electron Configuration Use a chart such as the one below. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. A horizontal row in the periodic. Members of a group typically have similar properties and electron configurations in their outer shell. ← electronic configurations of elements. Bromine is intermediate in reactivity between chlorine. Br (bromine) is an element with position number. This can be shortened to [ar]4s23d104p5. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine (br) is a chemical element. Most common application of bromine. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. The atomic number of bromine is 35. Use a chart such as the one below.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Electron Configuration Bromine (br) is a chemical element. The atomic number of bromine is 35. ← electronic configurations of elements. Use a chart such as the one below. A horizontal row in the periodic. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p. Bromine Atom Electron Configuration.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Atom Electron Configuration The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Most common application of bromine. A horizontal row in the periodic. Bromine is intermediate in reactivity between. Bromine Atom Electron Configuration.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Atom Electron Configuration Members of a group typically have similar properties and electron configurations in their outer shell. The atomic number of bromine is 35. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Most common application of bromine. ← electronic configurations of elements. This can be shortened to [ar]4s23d104p5. The bromine. Bromine Atom Electron Configuration.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Electron Configuration Members of a group typically have similar properties and electron configurations in their outer shell. Use a chart such as the one below. ← electronic configurations of elements. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine (br) is a chemical element. This can be shortened to [ar]4s23d104p5. A horizontal row in the periodic. The atomic number of bromine is 35.. Bromine Atom Electron Configuration.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Atom Electron Configuration Members of a group typically have similar properties and electron configurations in their outer shell. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Br (bromine) is an element with position number. Use. Bromine Atom Electron Configuration.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image Bromine Atom Electron Configuration The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Most common application of bromine. Bromine (br) is a chemical element. A horizontal row in the periodic. The atomic number of bromine is 35. Bromine is intermediate in reactivity between chlorine. Electron configuration of bromine is [ar] 3d10 4s2 4p5. This can be shortened to [ar]4s23d104p5. Br (bromine) is an element with position. Bromine Atom Electron Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Electron Configuration Bromine is intermediate in reactivity between chlorine. Use a chart such as the one below. Most common application of bromine. Members of a group typically have similar properties and electron configurations in their outer shell. Bromine (br) is a chemical element. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6. Bromine Atom Electron Configuration.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Bromine Atom Electron Configuration The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Most common application of bromine. A horizontal row in the periodic. ← electronic configurations of elements. Electron configuration of bromine is [ar] 3d10 4s2. Bromine Atom Electron Configuration.
From www.numerade.com
SOLVEDWhat is the electron configuration of a bromine atom (You CAN Bromine Atom Electron Configuration Bromine is intermediate in reactivity between chlorine. Br (bromine) is an element with position number. Electron configuration of bromine is [ar] 3d10 4s2 4p5. The atomic number of bromine is 35. A horizontal row in the periodic. Use a chart such as the one below. Bromine (br) is a chemical element. The bromine electron configuration, denoted as [ar] 4s 2. Bromine Atom Electron Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine (br) is a chemical element. Bromine is intermediate in reactivity between chlorine.. Bromine Atom Electron Configuration.
From brainly.com
Which is the electron configuration for bromine? Bromine Atom Electron Configuration Bromine (br) is a chemical element. Use a chart such as the one below. ← electronic configurations of elements. Electron configuration chart of all elements is mentioned in the table below. Electron configuration of bromine is [ar] 3d10 4s2 4p5. This can be shortened to [ar]4s23d104p5. A horizontal row in the periodic. Br (bromine) is an element with position number.. Bromine Atom Electron Configuration.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine Atom Electron Configuration The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Electron configuration of bromine is [ar] 3d10 4s2 4p5. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Use a chart such as the one below.. Bromine Atom Electron Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Electron Configuration Most common application of bromine. Electron configuration of bromine is [ar] 3d10 4s2 4p5. The atomic number of bromine is 35. Members of a group typically have similar properties and electron configurations in their outer shell. Bromine (br) is a chemical element. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. The bromine electron configuration,. Bromine Atom Electron Configuration.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Atom Electron Configuration ← electronic configurations of elements. The atomic number of bromine is 35. Most common application of bromine. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. This can be shortened to [ar]4s23d104p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases. Bromine Atom Electron Configuration.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Atom Electron Configuration Br (bromine) is an element with position number. The atomic number of bromine is 35. Electron configuration of bromine is [ar] 3d10 4s2 4p5. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Members of a group typically have similar properties and electron configurations in their outer shell. This can be shortened to [ar]4s23d104p5. Bromine is intermediate in reactivity between chlorine. A. Bromine Atom Electron Configuration.
From www.shutterstock.com
Quantum Numbers Over 3,097 RoyaltyFree Licensable Stock Vectors Bromine Atom Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Br (bromine) is an element with position number. This can be shortened to [ar]4s23d104p5. The atomic number of bromine is 35. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5.. Bromine Atom Electron Configuration.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. ← electronic configurations of elements. Electron configuration chart of all elements is mentioned in the table below. The atomic number of bromine is 35. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Br (bromine) is an element with position number. Members of a group typically have similar properties and electron. Bromine Atom Electron Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Electron Configuration Bromine (br) is a chemical element. Electron configuration of bromine is [ar] 3d10 4s2 4p5. ← electronic configurations of elements. This can be shortened to [ar]4s23d104p5. Br (bromine) is an element with position number. Most common application of bromine. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2. Bromine Atom Electron Configuration.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Bromine Atom Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. A horizontal row in the periodic. Use a chart such as the one below. Br (bromine) is an element with position number. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Most common application of bromine. ← electronic configurations of elements. Members of a group typically have similar properties and electron. Bromine Atom Electron Configuration.
From www.alamy.com
Bromine atom, with mass and energy levels. Vector illustration Stock Bromine Atom Electron Configuration Bromine is intermediate in reactivity between chlorine. Use a chart such as the one below. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. ← electronic configurations of elements. The electron configuration of. Bromine Atom Electron Configuration.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Bromine Atom Electron Configuration A horizontal row in the periodic. Bromine is intermediate in reactivity between chlorine. The atomic number of bromine is 35. Use a chart such as the one below. ← electronic configurations of elements. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. This can be shortened to [ar]4s23d104p5. Bromine (br) is a chemical element. Br (bromine) is an element with position number. Bromine Atom Electron Configuration.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine Atom Electron Configuration Br (bromine) is an element with position number. The atomic number of bromine is 35. Bromine is intermediate in reactivity between chlorine. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Most common application of bromine. A horizontal row in the periodic. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Members of a group typically have similar properties and electron configurations. Bromine Atom Electron Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Electron Configuration Br (bromine) is an element with position number. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Most common application of bromine. Use a chart such as the one below. ← electronic configurations. Bromine Atom Electron Configuration.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Atom Electron Configuration Electron configuration chart of all elements is mentioned in the table below. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Use a chart such as the one below. ← electronic configurations of elements. Bromine (br) is a chemical element. A horizontal row in the periodic. Br (bromine) is an element with position number. This can be shortened to [ar]4s23d104p5. The shorthand. Bromine Atom Electron Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Electron Configuration Most common application of bromine. Bromine is intermediate in reactivity between chlorine. Electron configuration chart of all elements is mentioned in the table below. Members of a group typically have similar properties and electron configurations in their outer shell. Br (bromine) is an element with position number. This can be shortened to [ar]4s23d104p5. The bromine electron configuration, denoted as [ar]. Bromine Atom Electron Configuration.
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Bromine Atom Electron Configuration ← electronic configurations of elements. Most common application of bromine. Electron configuration chart of all elements is mentioned in the table below. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. The atomic. Bromine Atom Electron Configuration.
From lambdageeks.com
Bromine Electron Configuration 7 Easy Steps on How to Write Bromine Atom Electron Configuration Electron configuration of bromine is [ar] 3d10 4s2 4p5. Bromine is intermediate in reactivity between chlorine. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine (br) is a chemical element. Members of. Bromine Atom Electron Configuration.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine Atom Electron Configuration The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. The shorthand electron configuration (or noble gas configuration) as well as. ← electronic configurations of elements. Bromine is intermediate in reactivity between chlorine. Members. Bromine Atom Electron Configuration.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Atom Electron Configuration This can be shortened to [ar]4s23d104p5. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Members of a group typically have similar properties and electron configurations in their outer shell. Use a chart such as the one below. Most common application of bromine. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2. Bromine Atom Electron Configuration.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Bromine Atom Electron Configuration The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Electron configuration chart of all elements is mentioned in the table below. The atomic number of bromine is 35. Electron configuration of bromine is. Bromine Atom Electron Configuration.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Atom Electron Configuration Br (bromine) is an element with position number. Bromine is intermediate in reactivity between chlorine. Bromine (br) is a chemical element. Members of a group typically have similar properties and electron configurations in their outer shell. This can be shortened to [ar]4s23d104p5. The atomic number of bromine is 35. Electron configuration chart of all elements is mentioned in the table. Bromine Atom Electron Configuration.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Atom Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. The atomic number of bromine is 35. A horizontal row in the periodic. Br (bromine) is an element with position number. ← electronic configurations of elements. Members of a group typically have similar properties and electron configurations in their outer shell. This can be shortened to [ar]4s23d104p5. Electron configuration. Bromine Atom Electron Configuration.
From 123bike.biz
bromine electron configuration DrBeckmann Bromine Atom Electron Configuration Br (bromine) is an element with position number. Bromine is intermediate in reactivity between chlorine. The atomic number of bromine is 35. Bromine (br) is a chemical element. This can be shortened to [ar]4s23d104p5. ← electronic configurations of elements. Electron configuration of bromine is [ar] 3d10 4s2 4p5. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The shorthand electron configuration (or. Bromine Atom Electron Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Electron Configuration This can be shortened to [ar]4s23d104p5. Bromine is intermediate in reactivity between chlorine. The atomic number of bromine is 35. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Bromine (br) is a chemical element. Use a chart such as the one below. Br (bromine) is an element with position number. The shorthand electron configuration (or noble gas configuration) as. Bromine Atom Electron Configuration.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Atom Electron Configuration Br (bromine) is an element with position number. Members of a group typically have similar properties and electron configurations in their outer shell. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. The atomic number of bromine is 35. A horizontal row in the periodic. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Electron. Bromine Atom Electron Configuration.