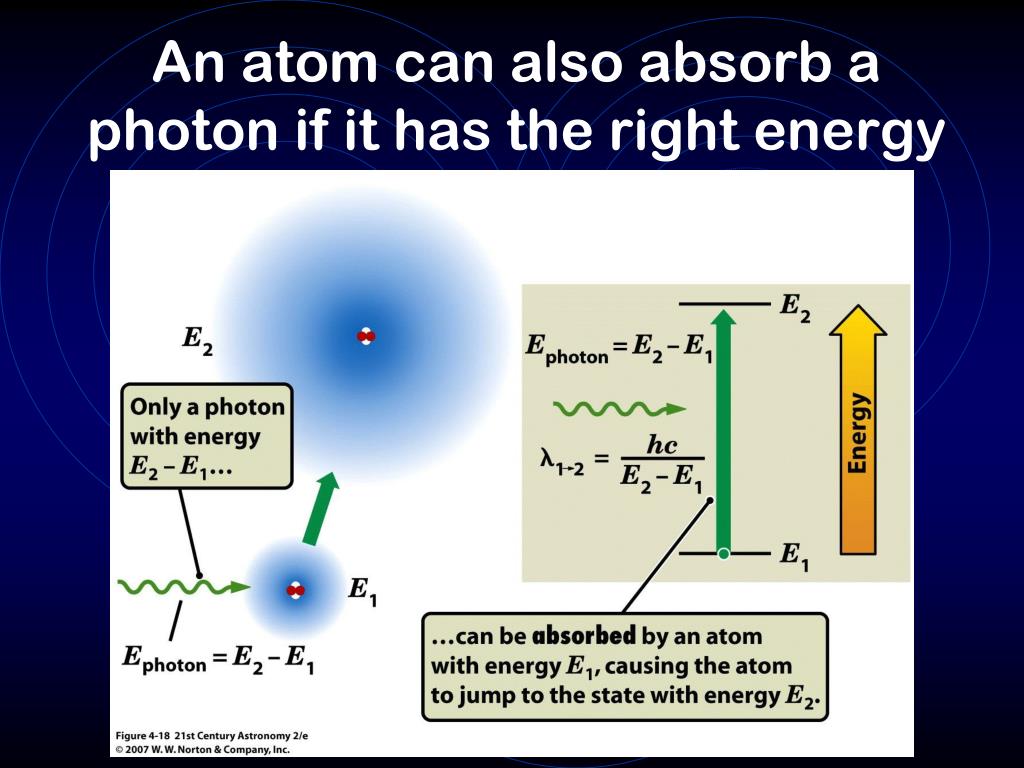
Can A Photon Absorb Energy . an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. When the photon is absorbed, the system gains energy. electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. Violet flowers absorb red and. photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of light. the atomic system supports multiple energy levels; However, the electron will eventually return to its. for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed.
from www.slideserve.com
Violet flowers absorb red and. photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of light. for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. the atomic system supports multiple energy levels; electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). When the photon is absorbed, the system gains energy. single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. However, the electron will eventually return to its. an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another.
PPT Chapter 5 Light PowerPoint Presentation, free download ID2767791
Can A Photon Absorb Energy Violet flowers absorb red and. an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. However, the electron will eventually return to its. photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of light. single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. the atomic system supports multiple energy levels; Violet flowers absorb red and. electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). When the photon is absorbed, the system gains energy.
From physics.stackexchange.com
quantum mechanics How much electrons can absorb a single photon Can A Photon Absorb Energy electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. the atomic system supports multiple energy levels; However, the electron will eventually. Can A Photon Absorb Energy.
From slideplayer.com
Light and Color light has both wave and particle characteristics ppt Can A Photon Absorb Energy electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. However, the electron will eventually return to its. for example, if a red photon of. Can A Photon Absorb Energy.
From www.youtube.com
Can a free electron absorb a photon? YouTube Can A Photon Absorb Energy electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. for example, if a red photon of frequency f encounters a molecule that has an energy. Can A Photon Absorb Energy.
From milagroskruwluna.blogspot.com
Describe How Plants Absorb Photons of Light Energy. MilagroskruwLuna Can A Photon Absorb Energy single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. However, the electron will eventually return to its. When the photon. Can A Photon Absorb Energy.
From byjus.com
when an electron in hydrogen atom absorb a photon of energy 12.75eV its Can A Photon Absorb Energy single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. for example, if a red photon of frequency f encounters. Can A Photon Absorb Energy.
From hanoverk12.web.fc2.com
Which electron jump in a hydrogen atom absorbs the photon of highest Can A Photon Absorb Energy electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). However, the electron will eventually return to its. the atomic system supports multiple energy levels; for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon. Can A Photon Absorb Energy.
From www.slideserve.com
PPT Chapter 5 Light PowerPoint Presentation, free download ID2768132 Can A Photon Absorb Energy an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. When the photon is absorbed, the system gains. Can A Photon Absorb Energy.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube Can A Photon Absorb Energy the atomic system supports multiple energy levels; When the photon is absorbed, the system gains energy. an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. Violet flowers absorb red and. single atoms can absorb energy from a photon and store it in an. Can A Photon Absorb Energy.
From www.slideserve.com
PPT PHOTOSYNTHESIS PowerPoint Presentation, free download ID1710862 Can A Photon Absorb Energy single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. When the photon is absorbed, the system gains energy. the atomic system supports multiple energy levels; photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of. Can A Photon Absorb Energy.
From www.scienceabc.com
Where Do The Photons Produced By A Source Of Light Come From? » ScienceABC Can A Photon Absorb Energy for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. Violet flowers absorb red and. When the photon is absorbed, the system gains energy. photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of. Can A Photon Absorb Energy.
From www.numerade.com
SOLVED Example 4; hydrogen electron absorbs energy and gets excited to Can A Photon Absorb Energy for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. Violet flowers absorb red and. However, the electron will eventually return to its. the atomic system supports multiple energy levels; electrons can transfer to a higher energy level by absorbing. Can A Photon Absorb Energy.
From www.physicsforums.com
Can photovoltaic cells capture gamma photons? Can A Photon Absorb Energy single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). However, the electron will eventually return to its. an atom can absorb or emit one. Can A Photon Absorb Energy.
From ar.inspiredpencil.com
Electron Energy Levels Photon Can A Photon Absorb Energy an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. However, the electron will eventually return to its. Violet flowers absorb red and. single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right. Can A Photon Absorb Energy.
From chem.libretexts.org
7.1 Vocabulary Chemistry LibreTexts Can A Photon Absorb Energy an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. Violet flowers absorb red and. However, the electron will eventually return to its. When the photon is absorbed, the system gains energy. the atomic system supports multiple energy levels; photons are the smallest possible. Can A Photon Absorb Energy.
From www.chegg.com
Solved When a hydrogen atom absorbs a photon of Can A Photon Absorb Energy the atomic system supports multiple energy levels; an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed.. Can A Photon Absorb Energy.
From www.slideserve.com
PPT Chapter 5 Light PowerPoint Presentation, free download ID2767791 Can A Photon Absorb Energy the atomic system supports multiple energy levels; Violet flowers absorb red and. single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. When the photon is absorbed, the system gains energy. electrons can transfer to a higher energy level by absorbing energy (usually. Can A Photon Absorb Energy.
From slideplayer.com
Modern Physics. ppt download Can A Photon Absorb Energy the atomic system supports multiple energy levels; However, the electron will eventually return to its. for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. When the photon is absorbed, the system gains energy. an atom can absorb or emit. Can A Photon Absorb Energy.
From www.bio.miami.edu
BIL 226 Lecture 10 Can A Photon Absorb Energy the atomic system supports multiple energy levels; When the photon is absorbed, the system gains energy. However, the electron will eventually return to its. for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. Violet flowers absorb red and. single. Can A Photon Absorb Energy.
From slideplayer.com
An Wave ppt download Can A Photon Absorb Energy the atomic system supports multiple energy levels; an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). single atoms can absorb energy from a photon. Can A Photon Absorb Energy.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps Can A Photon Absorb Energy Violet flowers absorb red and. the atomic system supports multiple energy levels; single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). When the photon. Can A Photon Absorb Energy.
From book.bionumbers.org
» How much energy is carried by photons used in photosynthesis? Can A Photon Absorb Energy Violet flowers absorb red and. When the photon is absorbed, the system gains energy. However, the electron will eventually return to its. single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. for example, if a red photon of frequency f encounters a molecule. Can A Photon Absorb Energy.
From www.chegg.com
Solved Which of the following complexes will absorb a photon Can A Photon Absorb Energy electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). However, the electron will eventually return to its. When the photon is absorbed, the system gains energy. photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of light. an atom can absorb. Can A Photon Absorb Energy.
From giovanniyouthmichael.blogspot.com
Describe How Plants Absorb Photons of Light Energy Can A Photon Absorb Energy the atomic system supports multiple energy levels; an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of light. Violet flowers absorb red and. single atoms can. Can A Photon Absorb Energy.
From slideplayer.com
CHAPTER 8 LECTURE SLIDES ppt download Can A Photon Absorb Energy the atomic system supports multiple energy levels; an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. When the photon is absorbed, the system gains energy. Violet flowers absorb red and. However, the electron will eventually return to its. single atoms can absorb energy. Can A Photon Absorb Energy.
From energywavetheory.com
Photon Creation and Absorption EWT Can A Photon Absorb Energy an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. the atomic system supports multiple energy levels; Violet flowers absorb. Can A Photon Absorb Energy.
From energywavetheory.com
Photon Creation and Absorption EWT Can A Photon Absorb Energy Violet flowers absorb red and. electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). the atomic system supports multiple energy levels; single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. When the photon. Can A Photon Absorb Energy.
From imathworks.com
Photon Absorption Why Can’t Electrons Absorb Any Energy and Emit the Can A Photon Absorb Energy an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. photons are the smallest possible particles of. Can A Photon Absorb Energy.
From slideplayer.com
What’s an XRay Ionization. ppt download Can A Photon Absorb Energy photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of light. However, the electron will eventually return to its. an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. electrons can transfer to a higher energy level. Can A Photon Absorb Energy.
From www.slideshare.net
Photosynthesis Can A Photon Absorb Energy for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. When the photon is absorbed, the system. Can A Photon Absorb Energy.
From studylib.net
Absorption / Emission of Photons and Conservation of Energy Can A Photon Absorb Energy for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. the atomic system supports multiple energy levels; an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another.. Can A Photon Absorb Energy.
From www.jobilize.com
What happens when a compound absorbs a photon of light By OpenStax Can A Photon Absorb Energy When the photon is absorbed, the system gains energy. Violet flowers absorb red and. the atomic system supports multiple energy levels; an atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another. However, the electron will eventually return to its. for example, if a red. Can A Photon Absorb Energy.
From energywavetheory.com
What is Quantum? EWT Can A Photon Absorb Energy electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). However, the electron will eventually return to its. single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. for example, if a red photon of. Can A Photon Absorb Energy.
From energywavetheory.com
Photon Creation and Absorption EWT Can A Photon Absorb Energy Violet flowers absorb red and. However, the electron will eventually return to its. for example, if a red photon of frequency f encounters a molecule that has an energy step, δe, equal to hf, then the photon can be absorbed. the atomic system supports multiple energy levels; single atoms can absorb energy from a photon and store. Can A Photon Absorb Energy.
From www.youtube.com
Why can't a free electron absorb a photon? YouTube Can A Photon Absorb Energy single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of. Violet flowers absorb red and. However, the electron will eventually return to its. When the photon is absorbed, the system gains energy. the atomic system supports multiple energy levels; an atom can absorb. Can A Photon Absorb Energy.
From rwu.pressbooks.pub
Chapter 12. Photosynthesis Introduction to Molecular and Cell Biology Can A Photon Absorb Energy electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). photons are the smallest possible particles of electromagnetic energy and therefore also the smallest possible particles of light. single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the. Can A Photon Absorb Energy.