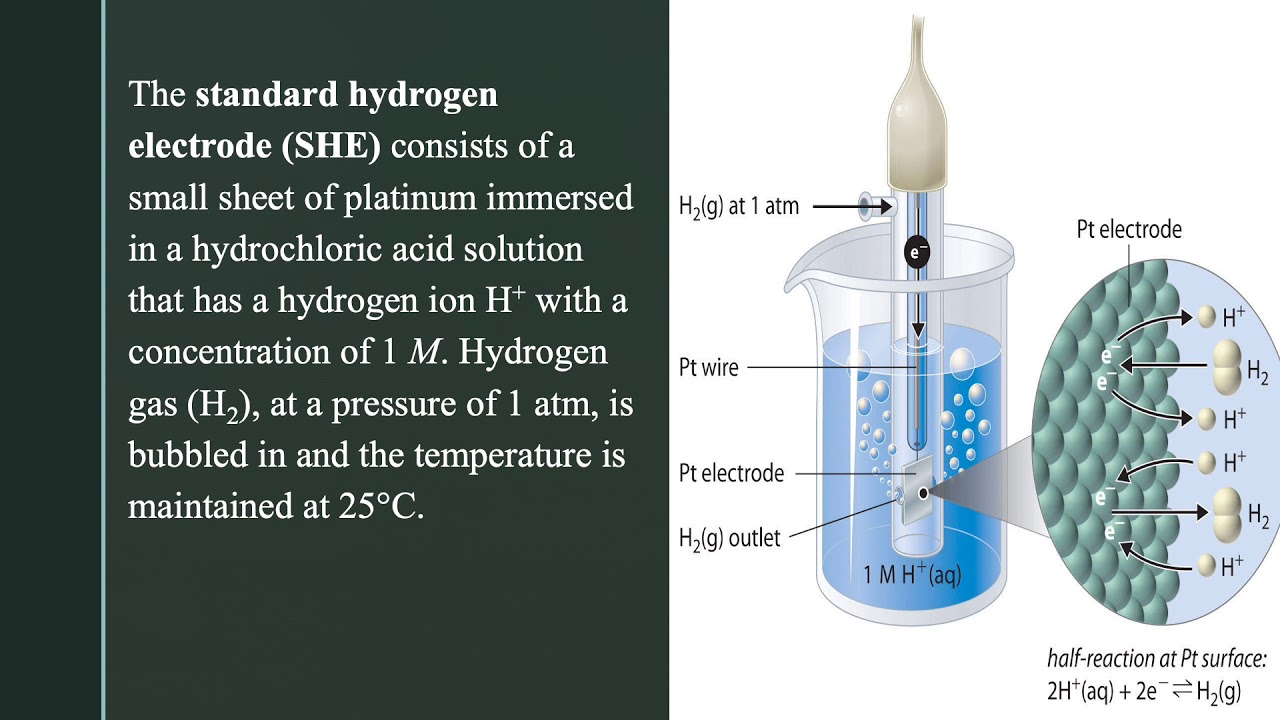
Standard Hydrogen Electrode Conditions . learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. Find out what a standard hydrogen electrode is and how to. See a labelled diagram of she and a solved example problem with the nernst equation. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials.
from www.youtube.com
See a labelled diagram of she and a solved example problem with the nernst equation. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. Find out what a standard hydrogen electrode is and how to.
Define standard hydrogen electrode SHE and write the reactions that
Standard Hydrogen Electrode Conditions the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. Find out what a standard hydrogen electrode is and how to. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. See a labelled diagram of she and a solved example problem with the nernst equation. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential.
From www.youtube.com
Standard hydrogen Electrode, Normal hydrogen Electrode Standard Hydrogen Electrode Conditions the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. See a labelled diagram of she and a solved example problem with the nernst equation. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. Find out what a standard hydrogen electrode is and how to. conditions,. Standard Hydrogen Electrode Conditions.
From www.slideserve.com
PPT STANDARD REDUCTION POTENTIAL PowerPoint Presentation, free Standard Hydrogen Electrode Conditions See a labelled diagram of she and a solved example problem with the nernst equation. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Find out what a standard hydrogen electrode is and how to. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on. Standard Hydrogen Electrode Conditions.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Conditions conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. learn how to compare the reactivity of metals using simple equilibria and. Standard Hydrogen Electrode Conditions.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Conditions See a labelled diagram of she and a solved example problem with the nernst equation. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. learn what a standard hydrogen electrode (she). Standard Hydrogen Electrode Conditions.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Standard Hydrogen Electrode Conditions conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. graphical representation of the onset potentials of the her/hor and oer/orr measured. Standard Hydrogen Electrode Conditions.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Conditions Find out what a standard hydrogen electrode is and how to. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. See a labelled diagram of she and. Standard Hydrogen Electrode Conditions.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Standard Hydrogen Electrode Conditions See a labelled diagram of she and a solved example problem with the nernst equation. Find out what a standard hydrogen electrode is and how to. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. graphical representation of the onset potentials of the her/hor and oer/orr measured. Standard Hydrogen Electrode Conditions.
From www.slideserve.com
PPT Cell Potentials at Standard Conditions PowerPoint Presentation Standard Hydrogen Electrode Conditions learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. Find out what a standard hydrogen electrode is and how to. See a. Standard Hydrogen Electrode Conditions.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Conditions graphical representation of the onset potentials of the her/hor and oer/orr measured using a. See a labelled diagram of she and a solved example problem with the nernst equation. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. Find out what a standard hydrogen electrode is and. Standard Hydrogen Electrode Conditions.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Conditions graphical representation of the onset potentials of the her/hor and oer/orr measured using a. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. learn what a standard hydrogen electrode (she). Standard Hydrogen Electrode Conditions.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Conditions conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Find out what a standard hydrogen electrode is. Standard Hydrogen Electrode Conditions.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Conditions conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. Find out what a standard hydrogen electrode is and how to. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. the choice that has been adopted is the standard hydrogen electrode, often. Standard Hydrogen Electrode Conditions.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Conditions learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. See a labelled diagram of she and a solved example problem with the nernst equation. Find out what. Standard Hydrogen Electrode Conditions.
From www.chegg.com
Solved what would the potential of a standard hydrogen Standard Hydrogen Electrode Conditions learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. Find out what a standard hydrogen electrode is and how to. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. graphical representation of the onset potentials. Standard Hydrogen Electrode Conditions.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Standard Hydrogen Electrode Conditions learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. See a labelled diagram of she and a solved example problem with the nernst equation. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. graphical representation. Standard Hydrogen Electrode Conditions.
From exoyfxrvd.blob.core.windows.net
Standard Hydrogen Electrode Nernst Equation at Silva Upchurch blog Standard Hydrogen Electrode Conditions See a labelled diagram of she and a solved example problem with the nernst equation. Find out what a standard hydrogen electrode is and how to. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. learn. Standard Hydrogen Electrode Conditions.
From www.youtube.com
DAT Reaction at Standard Hydrogen Electrode Explained YouTube Standard Hydrogen Electrode Conditions learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. Find out. Standard Hydrogen Electrode Conditions.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application Standard Hydrogen Electrode Conditions learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. See a labelled diagram of she and a solved example problem with the nernst equation. conditions, such. Standard Hydrogen Electrode Conditions.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Conditions learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. the choice. Standard Hydrogen Electrode Conditions.
From exomimdmo.blob.core.windows.net
Representation Of Standard Hydrogen Electrode at Rona Loomis blog Standard Hydrogen Electrode Conditions Find out what a standard hydrogen electrode is and how to. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. See a labelled diagram of she and a solved example problem with. Standard Hydrogen Electrode Conditions.
From scienceinfo.com
What is Standard Hydrogen Electrode? Standard Hydrogen Electrode Conditions graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Find out what a standard hydrogen electrode is and how to. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph. Standard Hydrogen Electrode Conditions.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Conditions conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. the choice that has been adopted is the standard hydrogen electrode, often. Standard Hydrogen Electrode Conditions.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Standard Hydrogen Electrode Conditions learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. See a labelled diagram of she and a solved example problem with the nernst equation. Find out what a standard hydrogen electrode is. Standard Hydrogen Electrode Conditions.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode Conditions learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. See a labelled diagram of she and a solved example problem with the nernst equation. Find out what a standard hydrogen electrode is and how to. graphical representation of the onset potentials of. Standard Hydrogen Electrode Conditions.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Conditions the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. conditions, such. Standard Hydrogen Electrode Conditions.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID4435141 Standard Hydrogen Electrode Conditions Find out what a standard hydrogen electrode is and how to. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. See a labelled diagram of she and a solved example problem with the nernst equation. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on. Standard Hydrogen Electrode Conditions.
From www.youtube.com
Standard Hydrogen Electrode Electrochemistry Chemistry Class 12 Standard Hydrogen Electrode Conditions graphical representation of the onset potentials of the her/hor and oer/orr measured using a. See a labelled diagram of she and a solved example problem with the nernst equation. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. Find out what a standard hydrogen electrode is and how to. learn how. Standard Hydrogen Electrode Conditions.
From www.chegg.com
Solved A standard hydrogen electrode (SHE) is shown in the Standard Hydrogen Electrode Conditions the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. See a labelled diagram of she and a solved example problem with the nernst equation. learn what a standard hydrogen electrode (she) is, how it is constructed, and. Standard Hydrogen Electrode Conditions.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Conditions learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Find out what. Standard Hydrogen Electrode Conditions.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Standard Hydrogen Electrode Conditions the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. See a labelled diagram of she and a solved example problem with the nernst equation. graphical representation of the onset potentials of the her/hor and oer/orr measured. Standard Hydrogen Electrode Conditions.
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode Conditions learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. Find out what a standard hydrogen electrode is and how to. graphical. Standard Hydrogen Electrode Conditions.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu Standard Hydrogen Electrode Conditions See a labelled diagram of she and a solved example problem with the nernst equation. learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. Find out what. Standard Hydrogen Electrode Conditions.
From cetfasly.blob.core.windows.net
Standard Hydrogen Electrodes Reaction at Haley Betton blog Standard Hydrogen Electrode Conditions See a labelled diagram of she and a solved example problem with the nernst equation. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. learn how to compare the reactivity of metals using simple equilibria and standard electrode potentials. learn what a standard hydrogen electrode (she). Standard Hydrogen Electrode Conditions.
From brainly.in
What is standard hydrogen electrode? How is it prepared? Explain with Standard Hydrogen Electrode Conditions learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. the choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph. Standard Hydrogen Electrode Conditions.
From www.researchgate.net
OCP measurements (electrode potential versus standard hydrogen Standard Hydrogen Electrode Conditions See a labelled diagram of she and a solved example problem with the nernst equation. conditions, such as the temperature (t), the pressure (p), and the electrolyte composition, concentration (c), and ph on its potential. Find out what a standard hydrogen electrode is and how to. the choice that has been adopted is the standard hydrogen electrode, often. Standard Hydrogen Electrode Conditions.