Catalytic Equation . This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. A catalyst accelerates a chemical reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. They do not appear in the reaction’s net equation and are not consumed during the reaction. What is the meaning and significance of. As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given temperature and thus the overall reaction rate. Catalysts participate in a chemical reaction and increase its rate. Catalysts are defined as substances that participate in a. A catalyst is a chemical substance. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalyst/substrate interactions and sabatier's principle. An overview of some basic concepts in catalysis.
from www.chegg.com
Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. A catalyst is a chemical substance. Catalysts are defined as substances that participate in a. They do not appear in the reaction’s net equation and are not consumed during the reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. An overview of some basic concepts in catalysis. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst accelerates a chemical reaction. Catalysts participate in a chemical reaction and increase its rate.
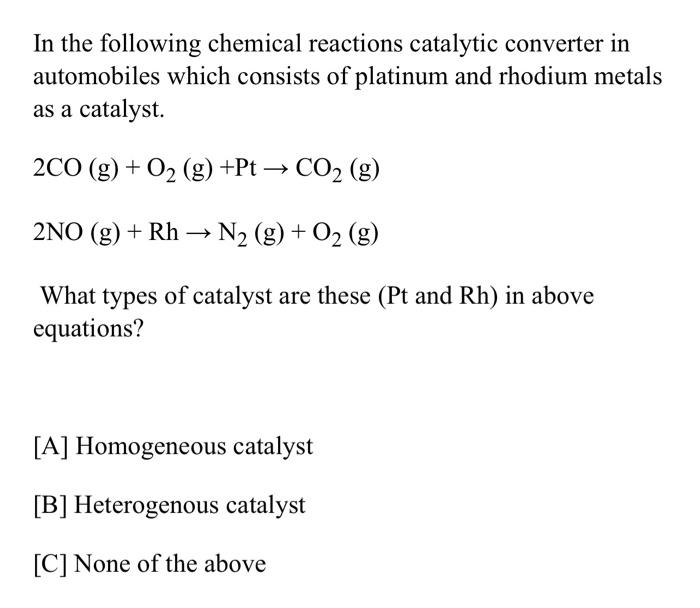
Solved In the following chemical reactions catalytic
Catalytic Equation A catalyst accelerates a chemical reaction. What is the meaning and significance of. A catalyst is a chemical substance. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. An overview of some basic concepts in catalysis. Catalysts are defined as substances that participate in a. Catalyst/substrate interactions and sabatier's principle. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given temperature and thus the overall reaction rate. Catalysts participate in a chemical reaction and increase its rate. Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. A catalyst accelerates a chemical reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalytic Equation Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst/substrate interactions and sabatier's principle. A catalyst accelerates a chemical reaction. An overview of some basic concepts in catalysis. A catalyst is a chemical substance.. Catalytic Equation.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalytic Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant,. Catalytic Equation.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalytic Equation A catalyst accelerates a chemical reaction. Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis. Catalytic Equation.
From www.mdpi.com
Processes Free FullText A Thermodynamic Analysis of Naphtha Catalytic Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. An overview of some basic concepts in catalysis. A catalyst is a chemical substance. Catalysts are defined as substances that participate in a. Catalysts allow a. Catalytic Equation.
From www.researchgate.net
Scheme 3. Chemical equation for the catalytic cleavage of the βO4 Catalytic Equation Catalysts are defined as substances that participate in a. Catalyst/substrate interactions and sabatier's principle. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. It does so by forming bonds with the reacting molecules, and. Catalytic Equation.
From www.slideserve.com
PPT Enzyme and Catalysis II PowerPoint Presentation, free Catalytic Equation A catalyst is a chemical substance. Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. A catalyst accelerates a chemical reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysts are defined as substances that participate in a. Catalysts participate in a chemical. Catalytic Equation.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID3206932 Catalytic Equation Catalyst/substrate interactions and sabatier's principle. Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. Catalysts are defined as substances that participate in a. What is the meaning and significance of. Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. A catalyst is a chemical substance. They do not appear in. Catalytic Equation.
From www.slideserve.com
PPT ENZYME BIOLOGICAL CATALYST PowerPoint Presentation, free Catalytic Equation Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst accelerates a chemical reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. An overview of some basic concepts in catalysis. What is the meaning and significance of. It does so by. Catalytic Equation.
From www.slideserve.com
PPT Option C2 (SL/HL) Part 2 Cracking PowerPoint Presentation, free Catalytic Equation It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. Catalyst/substrate interactions and sabatier's principle. As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given temperature and thus the overall reaction rate.. Catalytic Equation.
From www.chegg.com
Solved In the following chemical reactions catalytic Catalytic Equation It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. Catalysts are defined as substances that participate in a. They do not appear in the reaction’s net equation and are not consumed during the reaction. An overview of some basic concepts in catalysis. Catalyst/substrate interactions and sabatier's principle.. Catalytic Equation.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalytic Equation This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. As can be seen from the arrhenius equation,. Catalytic Equation.
From brainly.in
Equation for the catalytic oxidation of ammonia gas Brainly.in Catalytic Equation Catalysts are defined as substances that participate in a. An overview of some basic concepts in catalysis. They do not appear in the reaction’s net equation and are not consumed during the reaction. Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. It does so by forming bonds with the reacting molecules, and. Catalytic Equation.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Catalytic Equation Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst/substrate interactions and sabatier's principle. Catalysts are defined as substances that participate in a. What is the meaning and significance of. As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the. Catalytic Equation.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalytic Equation Catalysts participate in a chemical reaction and increase its rate. Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. Catalysts are defined as substances that participate in a. Catalyst/substrate interactions and sabatier's principle. A catalyst is a chemical substance. What is the meaning and significance of. Catalysis is the process that alters the. Catalytic Equation.
From www.researchgate.net
Dimensionless equations for catalytic reactions in discretized terms Catalytic Equation Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. An overview of some basic concepts in catalysis. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What is the meaning and significance of. Catalysts are defined as substances that participate in a. They do not appear in. Catalytic Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalytic Equation This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. They do not appear in the reaction’s net equation and are not consumed during the reaction. As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the. Catalytic Equation.
From www.slideserve.com
PPT Catalytic Converter PowerPoint Presentation, free download ID Catalytic Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a chemical substance. Catalysts participate in a chemical reaction and increase its rate. Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. It does so by forming bonds with the reacting molecules, and by allowing these to react. Catalytic Equation.
From exozrdcct.blob.core.windows.net
Equations In Catalytic Converters at Adam Hassel blog Catalytic Equation It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalyst/substrate interactions and sabatier's principle. Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. What is the meaning and. Catalytic Equation.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalytic Equation Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalyst/substrate interactions and sabatier's principle. It does so by forming bonds with the reacting. Catalytic Equation.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID5595643 Catalytic Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts are defined as substances that participate in a. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What is the meaning and significance of. An overview of some basic concepts in catalysis. Catalyst/substrate. Catalytic Equation.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalytic Equation Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst accelerates a chemical reaction. Catalyst/substrate interactions and sabatier's principle. As can be seen from the arrhenius equation, the magnitude of the activation energy,. Catalytic Equation.
From present5.com
Brief History of the Catalytic Converter The catalytic Catalytic Equation An overview of some basic concepts in catalysis. A catalyst accelerates a chemical reaction. What is the meaning and significance of. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate. Catalytic Equation.
From www.youtube.com
C.2 Catalytic reforming (SL) YouTube Catalytic Equation As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given temperature and thus the overall reaction rate. What is the meaning and significance of. Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalysts participate in a chemical reaction and increase. Catalytic Equation.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalytic Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant,. Catalytic Equation.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent Catalytic Equation This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. What is the meaning and significance of. Catalyst/substrate interactions and sabatier's principle. As can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at. Catalytic Equation.
From www.doubtnut.com
Write a balanced chemical equation for the reaction showing catalytic Catalytic Equation Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. Catalytic Equation.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalytic Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst accelerates a chemical reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance. As can be seen from the arrhenius equation, the magnitude of the activation energy,. Catalytic Equation.
From www.researchgate.net
a The reaction equations of 4 TZSM5MIL53(Al) catalytic system. b Catalytic Equation Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. They do not appear in the reaction’s net equation and are not consumed during the reaction. This page looks at the the different types of catalyst. Catalytic Equation.
From www.youtube.com
Write the balanced chemical equation for the catalytic oxidation of Catalytic Equation Define physisorption and chemisorption, and explain the role of the latter in initiating a catalytic event. A catalyst accelerates a chemical reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. They do not appear in the reaction’s net equation and are not consumed during. Catalytic Equation.
From www.mdpi.com
Materials Free FullText PROMETHEUS A CopperBased Polymetallic Catalytic Equation A catalyst is a chemical substance. A catalyst accelerates a chemical reaction. Catalysts participate in a chemical reaction and increase its rate. It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of. Catalytic Equation.
From www.youtube.com
Catalysts Chemistry Khan Academy YouTube Catalytic Equation What is the meaning and significance of. An overview of some basic concepts in catalysis. They do not appear in the reaction’s net equation and are not consumed during the reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts allow a reaction to. Catalytic Equation.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalytic Equation Catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts are defined. Catalytic Equation.
From brainly.in
define catalyst with example ?? Brainly.in Catalytic Equation It does so by forming bonds with the reacting molecules, and by allowing these to react to a product, which detaches from. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts participate in a chemical reaction and increase its rate. An overview of some basic concepts in catalysis. Catalysts. Catalytic Equation.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalytic Equation A catalyst is a chemical substance. Catalysts participate in a chemical reaction and increase its rate. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst accelerates a chemical reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation. Catalytic Equation.
From exozrdcct.blob.core.windows.net
Equations In Catalytic Converters at Adam Hassel blog Catalytic Equation This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst accelerates a chemical reaction. It does so by forming bonds with the reacting molecules, and by. Catalytic Equation.