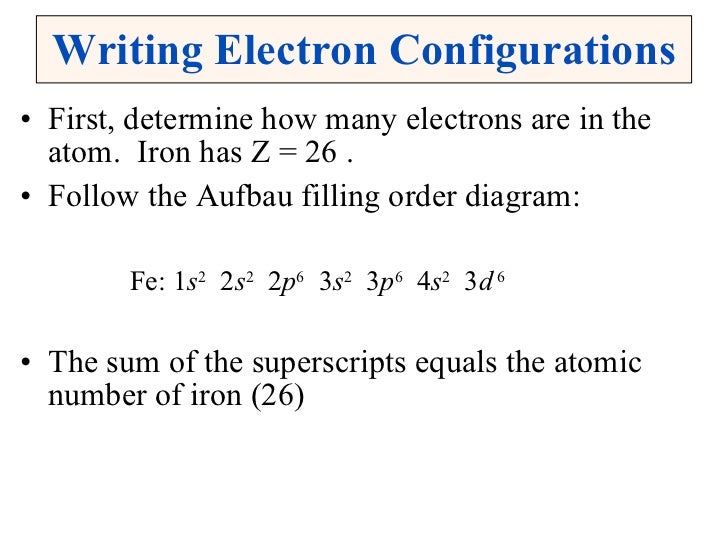
Shorthand Electron Configuration For Iron . Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The shorthand electron configuration (or noble gas configuration) as well as. Shorthand electron configuration of iron: It’s a transition metal from group 8 of the periodic table’s first transition series. Its valence orbitals are the #4s# and #3d# 's. This diagram shows how the electrons in the iron atom are. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Why should there be 26? 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? The following table lists the ionization energies ie (ionization. Electron configuration chart of all elements is mentioned in the table below. Writing the electron configuration, you really only. [ar] 3d 6 4s 2. The iron orbital diagram is a graphical representation of the electron configuration of the iron atom.
from nkppaperlfd.web.fc2.com
This diagram shows how the electrons in the iron atom are. The shorthand electron configuration (or noble gas configuration) as well as. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? Writing the electron configuration, you really only. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Why should there be 26? [ar] 3d 6 4s 2. Electron configuration chart of all elements is mentioned in the table below.
How to write shorthand electron configurations
Shorthand Electron Configuration For Iron Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. [ar] 3d 6 4s 2. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? The chemical element iron has the atomic number 26 and the symbol fe (from latin: Electron configuration chart of all elements is mentioned in the table below. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The shorthand electron configuration (or noble gas configuration) as well as. The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. Writing the electron configuration, you really only. Shorthand electron configuration of iron: Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. It’s a transition metal from group 8 of the periodic table’s first transition series. The following table lists the ionization energies ie (ionization. This diagram shows how the electrons in the iron atom are. Why should there be 26? Its valence orbitals are the #4s# and #3d# 's.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and Shorthand Electron Configuration For Iron Writing the electron configuration, you really only. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Its valence orbitals are the #4s# and #3d# 's. The following table lists the ionization energies ie (ionization. Shorthand electron configuration of iron: Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26. Shorthand Electron Configuration For Iron.
From guides.byjusweb.com
Electronic Configuration of Iron Fe element Valency, Applications Shorthand Electron Configuration For Iron This diagram shows how the electrons in the iron atom are. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? It’s a transition metal from group 8 of the periodic table’s first transition series. The shorthand electron configuration (or noble gas configuration) as well as. Its valence orbitals are the #4s# and #3d# 's. Shorthand electron. Shorthand Electron Configuration For Iron.
From nkppaperlfd.web.fc2.com
How to write shorthand electron configurations Shorthand Electron Configuration For Iron Why should there be 26? This diagram shows how the electrons in the iron atom are. The following table lists the ionization energies ie (ionization. Its valence orbitals are the #4s# and #3d# 's. Writing the electron configuration, you really only. [ar] 3d 6 4s 2. The chemical element iron has the atomic number 26 and the symbol fe (from. Shorthand Electron Configuration For Iron.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Shorthand Electron Configuration For Iron Shorthand electron configuration of iron: Why should there be 26? The shorthand electron configuration (or noble gas configuration) as well as. It’s a transition metal from group 8 of the periodic table’s first transition series. Its valence orbitals are the #4s# and #3d# 's. This diagram shows how the electrons in the iron atom are. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^. Shorthand Electron Configuration For Iron.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry for Shorthand Electron Configuration For Iron The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Electron configuration chart of all elements is mentioned in the table below. [ar] 3d 6 4s 2. Shorthand electron. Shorthand Electron Configuration For Iron.
From www.coursehero.com
[Solved] Write the noble gas shorthand configurations for the following Shorthand Electron Configuration For Iron Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The shorthand electron configuration (or noble gas configuration) as well as. This diagram shows how the electrons in the iron atom are. The following table lists the ionization energies ie (ionization. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number. Shorthand Electron Configuration For Iron.
From www.youtube.com
Electron Configuration Using Noble Gas Shorthand YouTube Shorthand Electron Configuration For Iron The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? [ar] 3d 6 4s 2. Writing the electron configuration, you really only. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an. Shorthand Electron Configuration For Iron.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic Shorthand Electron Configuration For Iron Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The shorthand electron configuration (or noble gas configuration) as well as. Why should there be 26? 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? It’s a transition metal from group 8 of the periodic table’s first transition series. The following table lists. Shorthand Electron Configuration For Iron.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Shorthand Electron Configuration For Iron Shorthand electron configuration of iron: Why should there be 26? [ar] 3d 6 4s 2. The following table lists the ionization energies ie (ionization. Writing the electron configuration, you really only. Electron configuration chart of all elements is mentioned in the table below. It’s a transition metal from group 8 of the periodic table’s first transition series. Its valence orbitals. Shorthand Electron Configuration For Iron.
From www.youtube.com
ShortHand Electron Configuration YouTube Shorthand Electron Configuration For Iron Electron configuration chart of all elements is mentioned in the table below. It’s a transition metal from group 8 of the periodic table’s first transition series. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. This diagram shows how the electrons. Shorthand Electron Configuration For Iron.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Shorthand Electron Configuration For Iron This diagram shows how the electrons in the iron atom are. The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. [ar] 3d 6 4s 2. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? It’s a transition metal from group 8 of the periodic table’s first transition series. Its. Shorthand Electron Configuration For Iron.
From www.numerade.com
SOLVED Question 1 1pts Consider a +2 cation of Iron There are Shorthand Electron Configuration For Iron [ar] 3d 6 4s 2. It’s a transition metal from group 8 of the periodic table’s first transition series. Shorthand electron configuration of iron: The following table lists the ionization energies ie (ionization. The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. Writing the electron configuration, you really only. 1s^ (2)2s^ (2)2p^ (6)3s^. Shorthand Electron Configuration For Iron.
From hra.animalia-life.club
Electron Configuration For Iron Shorthand Electron Configuration For Iron Electron configuration chart of all elements is mentioned in the table below. Shorthand electron configuration of iron: 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? Its valence orbitals are the #4s# and #3d# 's. It’s a transition metal from group 8 of the periodic table’s first transition series. Why should there be 26? Its core. Shorthand Electron Configuration For Iron.
From www.youtube.com
Electron Configuration of Transition Metals Iron Fe Cobalt Co and Shorthand Electron Configuration For Iron It’s a transition metal from group 8 of the periodic table’s first transition series. Why should there be 26? Shorthand electron configuration of iron: The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic. Shorthand Electron Configuration For Iron.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Shorthand Electron Configuration For Iron It’s a transition metal from group 8 of the periodic table’s first transition series. [ar] 3d 6 4s 2. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Shorthand electron configuration of iron: Writing the electron configuration, you really only. Its valence orbitals are the #4s# and #3d# 's. Electron configuration chart of all elements is. Shorthand Electron Configuration For Iron.
From hra.animalia-life.club
Electron Configuration For Iron Shorthand Electron Configuration For Iron This diagram shows how the electrons in the iron atom are. It’s a transition metal from group 8 of the periodic table’s first transition series. The shorthand electron configuration (or noble gas configuration) as well as. Writing the electron configuration, you really only. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Shorthand electron configuration of. Shorthand Electron Configuration For Iron.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Shorthand Electron Configuration For Iron The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. The following table lists the ionization energies ie (ionization. Shorthand electron configuration of iron: Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. This diagram shows how. Shorthand Electron Configuration For Iron.
From www.youtube.com
Electronic Configuration of Iron (Fe) YouTube Shorthand Electron Configuration For Iron Its valence orbitals are the #4s# and #3d# 's. Electron configuration chart of all elements is mentioned in the table below. Shorthand electron configuration of iron: Why should there be 26? This diagram shows how the electrons in the iron atom are. The following table lists the ionization energies ie (ionization. The shorthand electron configuration (or noble gas configuration) as. Shorthand Electron Configuration For Iron.
From vigglegiggle.weebly.com
Electrons and their Arrangement in Orbitals and in Ions vigglegiggle Shorthand Electron Configuration For Iron Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. This diagram shows how the electrons in the iron atom are. The following table lists the ionization energies ie (ionization. Shorthand electron configuration of iron: The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. The chemical element iron has the. Shorthand Electron Configuration For Iron.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID Shorthand Electron Configuration For Iron [ar] 3d 6 4s 2. Its valence orbitals are the #4s# and #3d# 's. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Its core orbitals are the. Shorthand Electron Configuration For Iron.
From www.youtube.com
3 54 Shorthand Electron Configurations YouTube Shorthand Electron Configuration For Iron 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Its valence orbitals are the #4s# and #3d# 's. This diagram shows how the electrons in the iron atom are. The iron orbital diagram is a graphical representation of the electron configuration of the. Shorthand Electron Configuration For Iron.
From www.showme.com
Shorthand Electron Configuration Electron Configuration ShowMe Shorthand Electron Configuration For Iron The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from group 8 of the periodic table’s first transition series. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? Its core. Shorthand Electron Configuration For Iron.
From sciencenotes.org
List of Electron Configurations of Elements Shorthand Electron Configuration For Iron Why should there be 26? The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? This diagram shows how the electrons in the iron atom are. The shorthand electron configuration (or noble gas configuration) as well as. The chemical element iron. Shorthand Electron Configuration For Iron.
From www.youtube.com
Shorthand Electron Configuration and Valence Electrons YouTube Shorthand Electron Configuration For Iron The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. Writing the electron configuration, you really only. The shorthand electron configuration (or noble gas configuration) as well as. This diagram shows how the electrons in the iron atom are. It’s a transition metal from group 8 of the periodic table’s first transition series. Its. Shorthand Electron Configuration For Iron.
From slideplayer.com
ELECTRON CONFIGURATION ppt download Shorthand Electron Configuration For Iron Electron configuration chart of all elements is mentioned in the table below. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Why should there be 26? [ar] 3d 6 4s. Shorthand Electron Configuration For Iron.
From ar.inspiredpencil.com
Periodic Table With Shorthand Electron Configuration Shorthand Electron Configuration For Iron Shorthand electron configuration of iron: Writing the electron configuration, you really only. Electron configuration chart of all elements is mentioned in the table below. Why should there be 26? [ar] 3d 6 4s 2. Its valence orbitals are the #4s# and #3d# 's. This diagram shows how the electrons in the iron atom are. Iron is a chemical element of. Shorthand Electron Configuration For Iron.
From ar.inspiredpencil.com
Electron Configuration Of Iron Shorthand Electron Configuration For Iron This diagram shows how the electrons in the iron atom are. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (6) are these 26 electrons here? Electron configuration chart of all elements is mentioned in the table below. The following table lists the ionization energies ie (ionization. Its valence orbitals are the #4s# and #3d# 's. [ar] 3d 6 4s 2. It’s. Shorthand Electron Configuration For Iron.
From sciencenotes.org
Noble Gas Configuration Shorthand Electron Configuration Shorthand Electron Configuration For Iron Shorthand electron configuration of iron: Writing the electron configuration, you really only. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. The shorthand electron configuration (or noble gas configuration) as well as. The chemical element iron has the atomic number. Shorthand Electron Configuration For Iron.
From www.slideserve.com
PPT Electron configurations tells us in which orbitals the electrons Shorthand Electron Configuration For Iron Why should there be 26? This diagram shows how the electrons in the iron atom are. The following table lists the ionization energies ie (ionization. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Writing the electron configuration, you really only. Shorthand electron. Shorthand Electron Configuration For Iron.
From www.youtube.com
A stepbystep description of how to write the electron configuration Shorthand Electron Configuration For Iron Its valence orbitals are the #4s# and #3d# 's. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. The shorthand electron configuration (or noble gas configuration) as well as. Writing the electron configuration, you really only. The chemical element iron has the atomic. Shorthand Electron Configuration For Iron.
From www.youtube.com
Shorthand Notation of Electron Configurations YouTube Shorthand Electron Configuration For Iron Why should there be 26? It’s a transition metal from group 8 of the periodic table’s first transition series. Writing the electron configuration, you really only. The chemical element iron has the atomic number 26 and the symbol fe (from latin: The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. Electron configuration chart. Shorthand Electron Configuration For Iron.
From www.youtube.com
Write a noble gas or shorthand electron configuration arsenic YouTube Shorthand Electron Configuration For Iron This diagram shows how the electrons in the iron atom are. The iron orbital diagram is a graphical representation of the electron configuration of the iron atom. The shorthand electron configuration (or noble gas configuration) as well as. Its valence orbitals are the #4s# and #3d# 's. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's.. Shorthand Electron Configuration For Iron.
From hra.animalia-life.club
Electron Configuration For Iron Shorthand Electron Configuration For Iron The following table lists the ionization energies ie (ionization. [ar] 3d 6 4s 2. This diagram shows how the electrons in the iron atom are. The shorthand electron configuration (or noble gas configuration) as well as. Writing the electron configuration, you really only. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Shorthand electron. Shorthand Electron Configuration For Iron.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Shorthand Electron Configuration For Iron Writing the electron configuration, you really only. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. The shorthand electron configuration (or noble gas configuration) as well as. Its valence orbitals. Shorthand Electron Configuration For Iron.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms Shorthand Electron Configuration For Iron Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is. Shorthand electron configuration of iron: Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. It’s a transition metal from group. Shorthand Electron Configuration For Iron.