What Happens To The Ph Of The Buffer As You Add Drops Of The Acid . A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. It is able to neutralize small amounts of added acid or base, thus. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. In other words, the buffer solution stops the acid and base from. They are diluted to the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn how buffers protect against ph changes when strong acid or base is added. It can resist changes in ph when strong acids or bases are added. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio.
from socratic.org
This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. It can resist changes in ph when strong acids or bases are added. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. They are diluted to the. Learn how buffers protect against ph changes when strong acid or base is added. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. It is able to neutralize small amounts of added acid or base, thus. In other words, the buffer solution stops the acid and base from. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
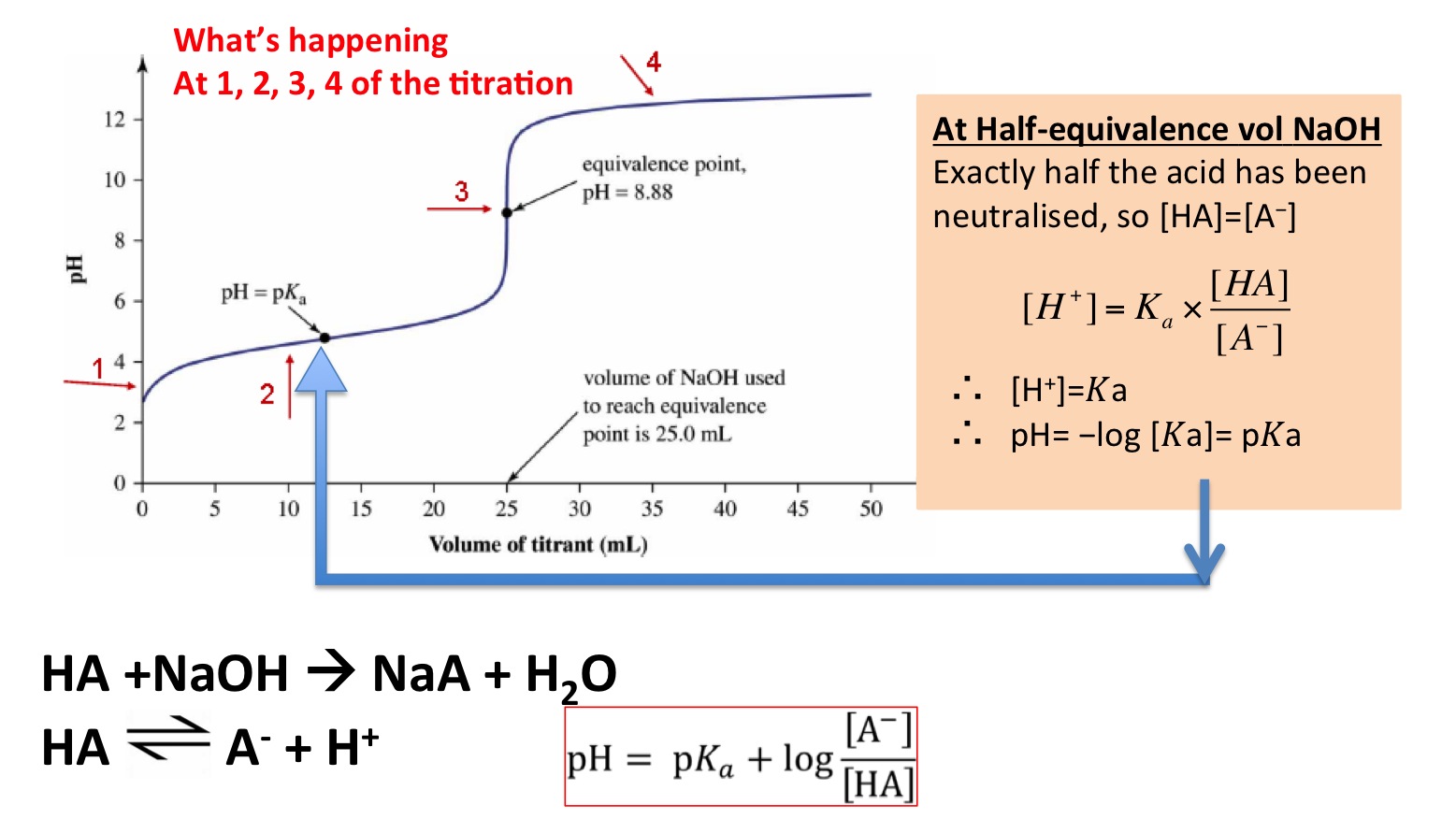
The "pH" at onehalf the equivalence point in an acidbase titration
What Happens To The Ph Of The Buffer As You Add Drops Of The Acid This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. Learn how buffers protect against ph changes when strong acid or base is added. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. It is able to neutralize small amounts of added acid or base, thus. It can resist changes in ph when strong acids or bases are added. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. They are diluted to the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In other words, the buffer solution stops the acid and base from. This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples.
From www.numerade.com
SOLVEDEffect of Acids and Bases on the pH of a Buffer Solution CI What Happens To The Ph Of The Buffer As You Add Drops Of The Acid It is able to neutralize small amounts of added acid or base, thus. Learn how buffers protect against ph changes when strong acid or base is added. They are diluted to the. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. In other words, the buffer solution stops the acid. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.numerade.com
SOLVED What happens to the pH of water as you add drops of a base What Happens To The Ph Of The Buffer As You Add Drops Of The Acid This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. They are diluted to the. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. When you add small quantities of an acid or alkali (base) to it, its ph does not change. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded What Happens To The Ph Of The Buffer As You Add Drops Of The Acid When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. In other words, the buffer solution stops the acid and base from. They are diluted to the. This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. A buffer is a solution that can resist ph. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From pdfprof.com
buffer capacity experiment procedure What Happens To The Ph Of The Buffer As You Add Drops Of The Acid When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. It is able to neutralize. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From courses.lumenlearning.com
Buffers Chemistry What Happens To The Ph Of The Buffer As You Add Drops Of The Acid It is able to neutralize small amounts of added acid or base, thus. They are diluted to the. It can resist changes in ph when strong acids or bases are added. In other words, the buffer solution stops the acid and base from. A buffer is a solution that can resist ph change upon the addition of an acidic or. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.numerade.com
SOLVED [6] What happens to the pH of the buffer as you add drops of What Happens To The Ph Of The Buffer As You Add Drops Of The Acid A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. In other words, the buffer solution stops the acid and base from. A buffer is a. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps What Happens To The Ph Of The Buffer As You Add Drops Of The Acid In other words, the buffer solution stops the acid and base from. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It can resist changes in ph when strong acids or bases are added. They are diluted to the. A buffer is a solution that contains a weak acid and. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG What Happens To The Ph Of The Buffer As You Add Drops Of The Acid Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. They are diluted to the.. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Happens To The Ph Of The Buffer As You Add Drops Of The Acid A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. It can resist changes in ph when strong acids or bases are added. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. Learn how buffers protect against ph. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.brainkart.com
Buffers What Happens To The Ph Of The Buffer As You Add Drops Of The Acid A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. They are diluted to the. Learn how buffers protect against ph changes when strong acid or base is added. When you add small. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.numerade.com
SOLVED A 25.0 mL sample of 0.30 M HCOOH is titrated with 0.25 M NaOH What Happens To The Ph Of The Buffer As You Add Drops Of The Acid In other words, the buffer solution stops the acid and base from. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. They are diluted to the. Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that contains. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.numerade.com
Calculate the pH of a buffer solution made from 0.30 M hydrofluoric What Happens To The Ph Of The Buffer As You Add Drops Of The Acid It is able to neutralize small amounts of added acid or base, thus. In other words, the buffer solution stops the acid and base from. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. If you look at the buffer formula, ph = pka + lg [salt]/. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens To The Ph Of The Buffer As You Add Drops Of The Acid A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. In other words, the buffer solution stops the acid and base from. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. It is able to neutralize small amounts. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.numerade.com
SOLVED [7] What happens to the pH of the water as you add drops of the What Happens To The Ph Of The Buffer As You Add Drops Of The Acid It is able to neutralize small amounts of added acid or base, thus. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. They are diluted to the. A. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.youtube.com
Find the pH of a Buffer after Adding HCl YouTube What Happens To The Ph Of The Buffer As You Add Drops Of The Acid They are diluted to the. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. It can resist changes in ph when strong acids or bases are added. In other words, the buffer solution stops the acid and base from. Learn how buffers protect against ph changes when. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.youtube.com
Find the pH of a Buffer after adding NaOH YouTube What Happens To The Ph Of The Buffer As You Add Drops Of The Acid They are diluted to the. In other words, the buffer solution stops the acid and base from. This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. It can resist changes in ph when strong acids or bases are added. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From study.com
Acidic, Basic & Neutral Solutions Overview, pH Scale & Uses Lesson What Happens To The Ph Of The Buffer As You Add Drops Of The Acid This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that contains a weak acid and its. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.expii.com
The pH of Weak Acid and Base Solutions — Examples Expii What Happens To The Ph Of The Buffer As You Add Drops Of The Acid They are diluted to the. Learn how buffers protect against ph changes when strong acid or base is added. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid.. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From byjus.com
When a small amount of HCL is added to a buffer solution of acetic acid What Happens To The Ph Of The Buffer As You Add Drops Of The Acid It is able to neutralize small amounts of added acid or base, thus. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. They are diluted to the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In other words, the. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Happens To The Ph Of The Buffer As You Add Drops Of The Acid A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. In other words, the buffer solution stops the acid and base from. A buffer is a solution that contains a weak acid. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.youtube.com
How to calculate the pH of a buffer solution YouTube What Happens To The Ph Of The Buffer As You Add Drops Of The Acid This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. It is able to neutralize small amounts of added acid or base, thus. Learn how buffers protect against ph changes when strong acid or base is added. When you add small quantities of an acid or alkali (base) to it, its ph does not change. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration What Happens To The Ph Of The Buffer As You Add Drops Of The Acid This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. It is able to neutralize small amounts of added acid or base, thus. In other words, the buffer solution stops the acid and base from. They. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.coursehero.com
[Solved] 1. Explain what is in a buffer. Discuss the function of a What Happens To The Ph Of The Buffer As You Add Drops Of The Acid It is able to neutralize small amounts of added acid or base, thus. In other words, the buffer solution stops the acid and base from. Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation What Happens To The Ph Of The Buffer As You Add Drops Of The Acid They are diluted to the. It is able to neutralize small amounts of added acid or base, thus. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that contains a weak. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube What Happens To The Ph Of The Buffer As You Add Drops Of The Acid A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In other words, the buffer solution stops the acid and base from. This tutorial explains the concept of buffer. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.numerade.com
SOLVED You add 10.00 mL of 0.100 M NaOH to 25.00 mL of pure water, and What Happens To The Ph Of The Buffer As You Add Drops Of The Acid If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.numerade.com
SOLVED Question 6 6.A student wishes to make a buffer by mixing 0 What Happens To The Ph Of The Buffer As You Add Drops Of The Acid Learn how buffers protect against ph changes when strong acid or base is added. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. They are diluted to the. It can resist. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.chemicals.co.uk
What is an Acid in Chemistry? The Chemistry Blog What Happens To The Ph Of The Buffer As You Add Drops Of The Acid A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. If you look at the buffer formula, ph =. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.slideshare.net
Ph and buffer What Happens To The Ph Of The Buffer As You Add Drops Of The Acid This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. It is able to neutralize small amounts of added acid or base, thus. Learn how buffers protect against ph changes when strong acid or base is added. They are diluted to the. It can resist changes in ph when strong acids or bases are added.. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.numerade.com
SOLVED Calculate the pH of a buffer solution that is made by mixing 80 What Happens To The Ph Of The Buffer As You Add Drops Of The Acid It is able to neutralize small amounts of added acid or base, thus. This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that can resist ph change upon the addition of an acidic or basic. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.osmosis.org
Making buffer solutions Osmosis What Happens To The Ph Of The Buffer As You Add Drops Of The Acid It is able to neutralize small amounts of added acid or base, thus. When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. It can resist changes in ph. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded What Happens To The Ph Of The Buffer As You Add Drops Of The Acid When you add small quantities of an acid or alkali (base) to it, its ph does not change significantly. A buffer is a solution that contains a weak acid and its conjugate base or a weak base and its conjugate acid. It is able to neutralize small amounts of added acid or base, thus. In other words, the buffer solution. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From design.udlvirtual.edu.pe
What Is The Ph Of A Buffer Solution Design Talk What Happens To The Ph Of The Buffer As You Add Drops Of The Acid A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In other words, the buffer solution stops the acid and base from. This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. If you look at the buffer formula, ph = pka + lg [salt]/ [acid],. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From byjus.com
For preparing a buffer of pH 6 by mixing sodium acetate and acetic acid What Happens To The Ph Of The Buffer As You Add Drops Of The Acid It can resist changes in ph when strong acids or bases are added. This tutorial explains the concept of buffer capacity, buffer ph, and buffer components with examples. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn how buffers protect against ph changes when strong acid or base is. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.
From lessonlibscherer.z21.web.core.windows.net
How To Calculate Ph What Happens To The Ph Of The Buffer As You Add Drops Of The Acid A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. In other words, the buffer solution stops the acid and base from. When you add small quantities of an. What Happens To The Ph Of The Buffer As You Add Drops Of The Acid.