Is Hx A Strong Acid . Hcl is a very strong acid, and hbr even more so. If you're seeing this message, it means we're having trouble loading external resources on our website. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. This is because it binds hydrogen much more. Hf is the only weak acid. When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. If you're behind a web filter, please. Hydrobromic acid and hydriodic acid as strong acids.
from www.numerade.com
When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. Hcl is a very strong acid, and hbr even more so. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). If you're behind a web filter, please. Hydrobromic acid and hydriodic acid as strong acids. This is because it binds hydrogen much more. If you're seeing this message, it means we're having trouble loading external resources on our website. Hf is the only weak acid. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does.
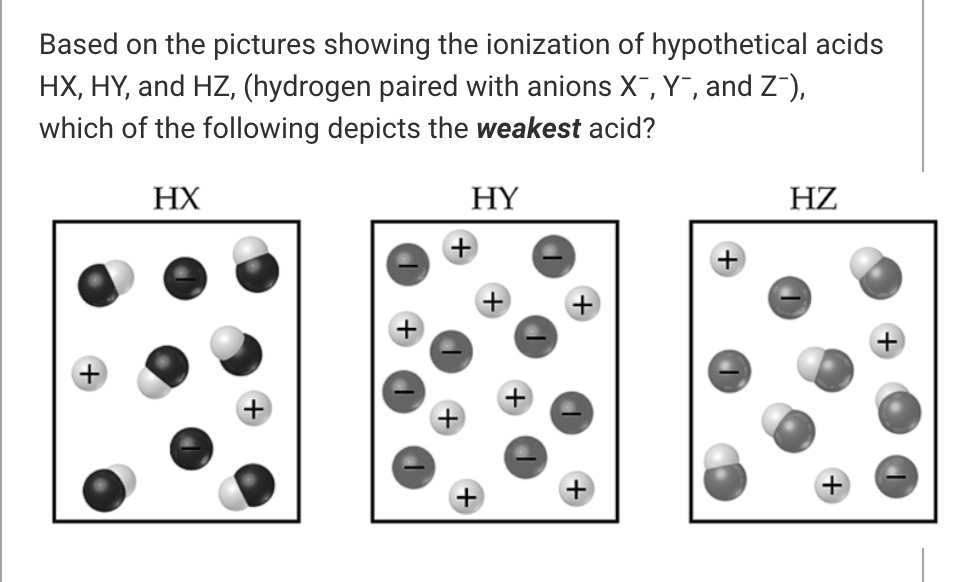
SOLVED Based on the pictures showing the ionization of hypothetical
Is Hx A Strong Acid By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. Hydrobromic acid and hydriodic acid as strong acids. Hf is the only weak acid. This is because it binds hydrogen much more. Hcl is a very strong acid, and hbr even more so. If you're seeing this message, it means we're having trouble loading external resources on our website. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. If you're behind a web filter, please.
From www.chegg.com
Solved HX The diagrams represent aqueous solutions of three Is Hx A Strong Acid If you're behind a web filter, please. Hcl is a very strong acid, and hbr even more so. This is because it binds hydrogen much more. When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes. Is Hx A Strong Acid.
From www.slideserve.com
PPT Common Strong acids PowerPoint Presentation, free download ID Is Hx A Strong Acid Hydrobromic acid and hydriodic acid as strong acids. Hcl is a very strong acid, and hbr even more so. If you're seeing this message, it means we're having trouble loading external resources on our website. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. This is because it binds. Is Hx A Strong Acid.
From www.numerade.com
SOLVED In each of the following diagrams of acid solutions determine Is Hx A Strong Acid Hcl is a very strong acid, and hbr even more so. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. Hydrobromic acid and hydriodic acid as strong acids. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). Hydrogen bromide and hydrogen iodide. Is Hx A Strong Acid.
From www.numerade.com
SOLVED Consider the generic acids HX, HY, and HZ. Which is the Is Hx A Strong Acid When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. If you're seeing this message, it means we're having trouble loading external resources on our website. Hydrobromic acid and hydriodic acid as strong acids. Hf is the only weak acid. This is because it binds hydrogen much more. For example, hydrochloric. Is Hx A Strong Acid.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Is Hx A Strong Acid Hcl is a very strong acid, and hbr even more so. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). This is because it binds hydrogen much more. Hf is the only weak acid. If you're seeing this message, it means we're having trouble loading external resources on our website.. Is Hx A Strong Acid.
From www.slideserve.com
PPT CHEM160 General Chemistry II Lecture Presentation Aqueous Is Hx A Strong Acid Hf is the only weak acid. If you're seeing this message, it means we're having trouble loading external resources on our website. Hydrobromic acid and hydriodic acid as strong acids. This is because it binds hydrogen much more. If you're behind a web filter, please. Hcl is a very strong acid, and hbr even more so. By definition, a strong. Is Hx A Strong Acid.
From www.chegg.com
Solved Arrange the three acids in order of decreasing value Is Hx A Strong Acid Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. If you're seeing this message, it means we're having trouble loading external resources on our website. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). By definition, a strong acid. Is Hx A Strong Acid.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo Is Hx A Strong Acid If you're behind a web filter, please. Hf is the only weak acid. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. When hydrogen chloride dissolves in water (to produce hydrochloric acid),. Is Hx A Strong Acid.
From slideplayer.com
Chemistry NCEA L3 3.6 Aqueous Systems ppt download Is Hx A Strong Acid If you're behind a web filter, please. This is because it binds hydrogen much more. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. By definition, a strong acid yields. Is Hx A Strong Acid.
From slideplayer.com
CHM 101 Sinex Acids and Bases Ch ppt download Is Hx A Strong Acid If you're seeing this message, it means we're having trouble loading external resources on our website. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. This is because it binds hydrogen much more. Hf is the only weak acid. For example, hydrochloric acid is a strong acid that ionizes essentially completely in. Is Hx A Strong Acid.
From www.numerade.com
SOLVED Part A Detemmine if the following diagrams represents strong Is Hx A Strong Acid When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. If you're seeing this message, it means we're having trouble loading external resources on our website. Hcl is a very strong acid, and hbr even more so. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the. Is Hx A Strong Acid.
From www.coursehero.com
[Solved] Consider two hypothetical acids a strong acid HX(aq) and a Is Hx A Strong Acid Hydrobromic acid and hydriodic acid as strong acids. This is because it binds hydrogen much more. When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. If you're behind a web filter, please. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen. Is Hx A Strong Acid.
From www.chegg.com
Solved The images below represent solutions of two acids, HX Is Hx A Strong Acid Hf is the only weak acid. This is because it binds hydrogen much more. If you're seeing this message, it means we're having trouble loading external resources on our website. Hcl is a very strong acid, and hbr even more so. Hydrobromic acid and hydriodic acid as strong acids. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\). Is Hx A Strong Acid.
From www.numerade.com
SOLVEDDetermine if each of the following diagrams represents a strong Is Hx A Strong Acid When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. If you're seeing this message, it means we're having trouble loading external resources on our website. Hydrobromic acid and hydriodic acid as strong acids. Hf is the only weak acid. Hcl is a very strong acid, and hbr even more so.. Is Hx A Strong Acid.
From www.numerade.com
SOLVED Based on the pictures showing the ionization of hypothetical Is Hx A Strong Acid If you're behind a web filter, please. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. Hf is the only weak acid. This is because it binds hydrogen much more. Hcl is. Is Hx A Strong Acid.
From www.numerade.com
SOLVED If acid HX is stronger than acid HY, which statements are true Is Hx A Strong Acid If you're behind a web filter, please. Hydrobromic acid and hydriodic acid as strong acids. Hcl is a very strong acid, and hbr even more so. If you're seeing this message, it means we're having trouble loading external resources on our website. Hf is the only weak acid. This is because it binds hydrogen much more. When hydrogen chloride dissolves. Is Hx A Strong Acid.
From slideplayer.com
Chapter 11 Alcohols and Ethers ppt download Is Hx A Strong Acid By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. If you're seeing this message, it means we're having trouble loading external resources on our website. Hcl is a very strong acid, and hbr even more so. Hydrobromic acid and hydriodic acid as strong acids. This is because it binds hydrogen much more.. Is Hx A Strong Acid.
From www.numerade.com
SOLVED 'multiple options please check all options The pH of a 0.01 M Is Hx A Strong Acid This is because it binds hydrogen much more. Hydrobromic acid and hydriodic acid as strong acids. If you're behind a web filter, please. Hcl is a very strong acid, and hbr even more so. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. For example, hydrochloric acid is a. Is Hx A Strong Acid.
From slideplayer.com
AcidBase Equilibria. ppt download Is Hx A Strong Acid Hf is the only weak acid. Hcl is a very strong acid, and hbr even more so. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). Hydrobromic acid and hydriodic acid as strong. Is Hx A Strong Acid.
From www.youtube.com
Trends in the Acid Strength of HX Binary Acids & Oxoacids YouTube Is Hx A Strong Acid When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. This is because it binds hydrogen much more. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. Hydrobromic acid and hydriodic acid as strong acids. By definition, a strong acid. Is Hx A Strong Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Is Hx A Strong Acid When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. Hf is the only weak. Is Hx A Strong Acid.
From www.transtutors.com
(Get Answer) Strong Acid HX H++ X Strong Base MOH Mt + OH Weak Is Hx A Strong Acid By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. Hf is the only weak acid. This is because it binds hydrogen much more. When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. Hydrobromic acid and hydriodic acid as strong acids. If you're. Is Hx A Strong Acid.
From www.chemistrysteps.com
Alcohol Reaction with HCl, HBr and HI Acids Chemistry Steps Is Hx A Strong Acid Hf is the only weak acid. If you're seeing this message, it means we're having trouble loading external resources on our website. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. Hydrogen bromide. Is Hx A Strong Acid.
From www.chegg.com
Solved 22. HX and H2Y are Arrhenius acids. Use the Is Hx A Strong Acid Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. This is because it binds hydrogen much more. If you're behind a web filter, please. Hcl is a very strong acid, and hbr. Is Hx A Strong Acid.
From slideplayer.com
AcidBase Equilibria The Nature of Acids and Bases ppt download Is Hx A Strong Acid If you're behind a web filter, please. Hcl is a very strong acid, and hbr even more so. Hf is the only weak acid. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react. Is Hx A Strong Acid.
From slideplayer.com
Chapter 17 buffers resist changes in pH by neutralizing added acid or Is Hx A Strong Acid Hcl is a very strong acid, and hbr even more so. If you're seeing this message, it means we're having trouble loading external resources on our website. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). This is because it binds hydrogen much more. Hf is the only weak acid.. Is Hx A Strong Acid.
From www.numerade.com
SOLVEDEqual quantities of the hypothetical strong acid HX, weak acid Is Hx A Strong Acid If you're behind a web filter, please. If you're seeing this message, it means we're having trouble loading external resources on our website. Hydrobromic acid and hydriodic acid as strong acids. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. Hcl is a very strong acid, and hbr even more so. This. Is Hx A Strong Acid.
From www.numerade.com
The following diagrams represent aqueous solutions of two monoprotic Is Hx A Strong Acid When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. Hcl is a very strong acid, and hbr even more so. This is because it binds hydrogen much more. Hf is the only weak acid. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution. Is Hx A Strong Acid.
From www.numerade.com
SOLVED The following diagrams represent aqueous solutions of two Is Hx A Strong Acid If you're seeing this message, it means we're having trouble loading external resources on our website. Hf is the only weak acid. Hcl is a very strong acid, and hbr even more so. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). Hydrobromic acid and hydriodic acid as strong acids.. Is Hx A Strong Acid.
From www.numerade.com
SOLVED Suppose you are comparing two acids, HX and HY If HX is a Is Hx A Strong Acid Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. If you're seeing this message, it means we're having trouble loading external resources on our website. Hf is the only weak acid. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. For. Is Hx A Strong Acid.
From slideplayer.com
CHM 101 Sinex Acids and Bases Ch ppt download Is Hx A Strong Acid Hydrobromic acid and hydriodic acid as strong acids. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). This is because it binds hydrogen much more. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. If you're seeing this message,. Is Hx A Strong Acid.
From www.numerade.com
SOLVED The following diagrams represent aqueous solutions of three Is Hx A Strong Acid By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. Hf is the only weak acid. Hcl is a very strong acid, and hbr even more so. Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. For example, hydrochloric acid is a. Is Hx A Strong Acid.
From www.turito.com
List of Top 7 Strong Acids Turito US Blog Is Hx A Strong Acid Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. Hcl is a very strong acid, and hbr even more so. If you're behind a web filter, please. This is because it binds hydrogen much more. Hf is the only weak acid. For example, hydrochloric acid is a strong acid. Is Hx A Strong Acid.
From www.slideserve.com
PPT Acids, Bases PowerPoint Presentation, free download ID1391470 Is Hx A Strong Acid Hcl is a very strong acid, and hbr even more so. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). Hf is the only weak acid. This is because it binds hydrogen much more. By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in. Is Hx A Strong Acid.
From www.numerade.com
The following diagrams represent aqueous solutions of two monoprotic Is Hx A Strong Acid When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost all the hydrogen chloride molecules react in this. For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce \(h_3o^+\). By definition, a strong acid yields 100% of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. This is because it binds. Is Hx A Strong Acid.