Dilution Equation Practice Problems . 0.19 m (the final volume is 900 ml, set up the. These dilution example problems show how to perform the calculations needed to make a diluted solution. You dilute the solution to 600 ml. How much of it do you need to prepare 50 ml of a. I add 560 ml more water to it? Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. The key idea behind a dilution is the number of moles of solute in. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. The concentration and the volumes change in a dilution. There’s a bottle of 0.750 m nacl on a shelf.
from www.youtube.com
The concentration and the volumes change in a dilution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. You dilute the solution to 600 ml. How much of it do you need to prepare 50 ml of a. These dilution example problems show how to perform the calculations needed to make a diluted solution. I add 560 ml more water to it? 0.19 m (the final volume is 900 ml, set up the. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. There’s a bottle of 0.750 m nacl on a shelf. The key idea behind a dilution is the number of moles of solute in.
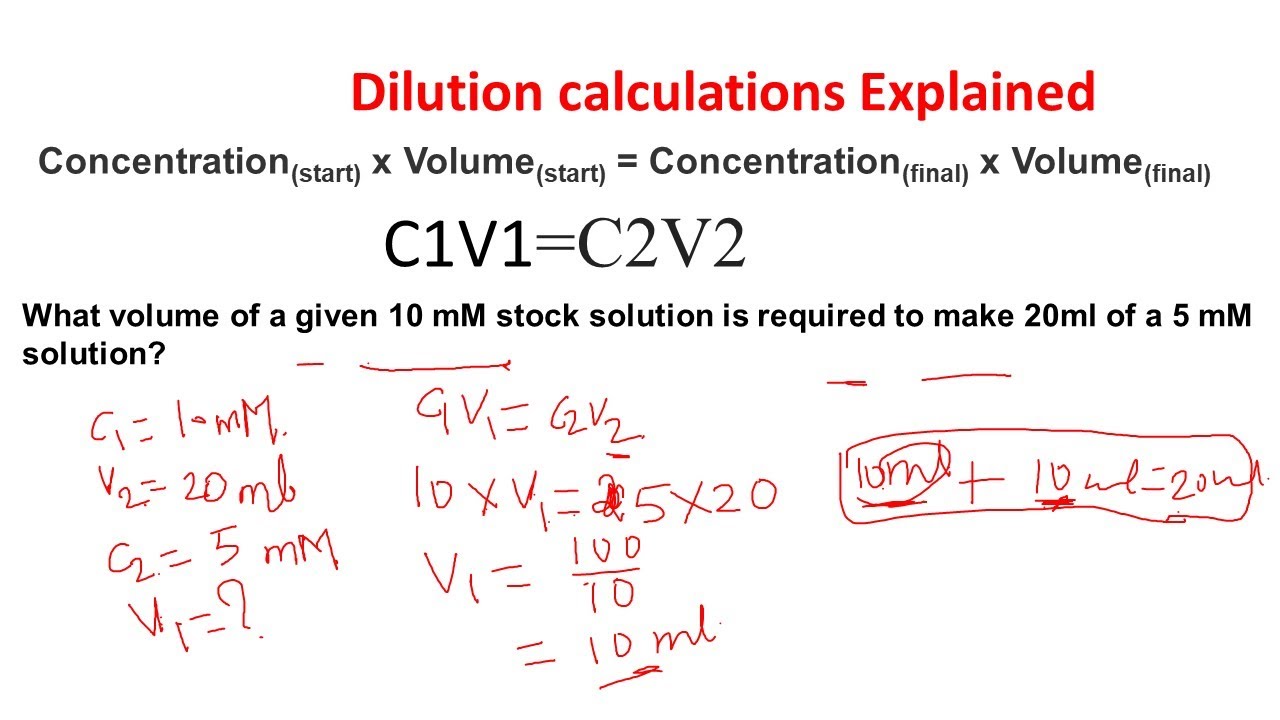
Dilution calculations Dilution problems Stock dilutions Biology and
Dilution Equation Practice Problems These dilution example problems show how to perform the calculations needed to make a diluted solution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. These dilution example problems show how to perform the calculations needed to make a diluted solution. I add 560 ml more water to it? How much of it do you need to prepare 50 ml of a. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. You dilute the solution to 600 ml. 0.19 m (the final volume is 900 ml, set up the. There’s a bottle of 0.750 m nacl on a shelf. The concentration and the volumes change in a dilution. The key idea behind a dilution is the number of moles of solute in. For example, we might say that a glass.
From www.chemistryworksheet.com
Dilution Problems Chemistry Worksheet With Answers Dilution Equation Practice Problems There’s a bottle of 0.750 m nacl on a shelf. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. The key idea behind a dilution is the number of moles of solute in. 0.19 m (the final volume is 900 ml, set up the. These dilution example problems show how to perform. Dilution Equation Practice Problems.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution Equation Practice Problems 0.19 m (the final volume is 900 ml, set up the. I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How much of it do you need to prepare 50 ml of a. Dilution is the process whereby the concentration of a solution. Dilution Equation Practice Problems.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution Equation Practice Problems The key idea behind a dilution is the number of moles of solute in. The concentration and the volumes change in a dilution. There’s a bottle of 0.750 m nacl on a shelf. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. I add 560 ml more water to it? For. Dilution Equation Practice Problems.
From www.chegg.com
Solved Practice Dilution Problems Section I 1. How much Dilution Equation Practice Problems 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 0.19 m (the final volume is 900 ml, set up the. There’s a bottle of 0.750 m nacl on a shelf. The key idea behind a dilution is the number of moles of solute in. You dilute the solution to 600 ml.. Dilution Equation Practice Problems.
From www.chegg.com
Solved Dilution Problems For problems 7 11 use the dilution Dilution Equation Practice Problems I add 560 ml more water to it? Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. You dilute the solution to 600 ml. There’s a bottle of 0.750 m nacl on a shelf. The concentration and the volumes change in a dilution. For example, we might say that a glass. The. Dilution Equation Practice Problems.
From www.youtube.com
Dilution Practice Problems 4 & 5 YouTube Dilution Equation Practice Problems I add 560 ml more water to it? 0.19 m (the final volume is 900 ml, set up the. You dilute the solution to 600 ml. The concentration and the volumes change in a dilution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How much of it do you need. Dilution Equation Practice Problems.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Equation Practice Problems The concentration and the volumes change in a dilution. How much of it do you need to prepare 50 ml of a. I add 560 ml more water to it? These dilution example problems show how to perform the calculations needed to make a diluted solution. The key idea behind a dilution is the number of moles of solute in.. Dilution Equation Practice Problems.
From www.chegg.com
Solved Dilution Problems 611 Use the dilution equation Dilution Equation Practice Problems Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. These dilution example problems show how to perform the calculations needed to make a diluted solution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of 0.750 m nacl on a. Dilution Equation Practice Problems.
From www.chegg.com
Solved This problem set should help you practice dilution Dilution Equation Practice Problems The concentration and the volumes change in a dilution. I add 560 ml more water to it? Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. There’s a bottle of 0.750 m nacl on a shelf. These dilution example problems show how to perform the calculations needed to make a diluted solution.. Dilution Equation Practice Problems.
From www.youtube.com
Gen Chem 1 Molarity and Dilution Practice Problems pt 1 YouTube Dilution Equation Practice Problems 0.19 m (the final volume is 900 ml, set up the. I add 560 ml more water to it? How much of it do you need to prepare 50 ml of a. For example, we might say that a glass. You dilute the solution to 600 ml. The key idea behind a dilution is the number of moles of solute. Dilution Equation Practice Problems.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Equation Practice Problems For example, we might say that a glass. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. How much of it do you need to prepare 50 ml of a. The concentration and the. Dilution Equation Practice Problems.
From www.youtube.com
Molarity Dilution Practice Problem 2 YouTube Dilution Equation Practice Problems You dilute the solution to 600 ml. There’s a bottle of 0.750 m nacl on a shelf. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. The key idea behind a dilution is the. Dilution Equation Practice Problems.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Equation Practice Problems Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. For example, we might say that a glass. The concentration and the volumes change in a dilution. These dilution example problems show how to perform the calculations needed to make a diluted solution. There’s a bottle of 0.750 m nacl on a shelf.. Dilution Equation Practice Problems.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Equation Practice Problems You dilute the solution to 600 ml. How much of it do you need to prepare 50 ml of a. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. The key idea behind a dilution is the number of moles of solute in. I add 560 ml more water to it? There’s. Dilution Equation Practice Problems.
From www.youtube.com
Molarity Dilution Practice Problem 3 YouTube Dilution Equation Practice Problems Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. There’s a bottle of 0.750 m nacl on a shelf. These dilution example problems show how to perform the calculations needed to make a diluted solution. For example, we might say that a glass. How much of it do you need to prepare. Dilution Equation Practice Problems.
From study.com
Quiz & Worksheet How to Calculate Dilution of Solutions Dilution Equation Practice Problems 0.19 m (the final volume is 900 ml, set up the. I add 560 ml more water to it? The concentration and the volumes change in a dilution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of 0.750 m nacl on a shelf. Dilution is the process. Dilution Equation Practice Problems.
From www.youtube.com
Solving dilution problems YouTube Dilution Equation Practice Problems The concentration and the volumes change in a dilution. There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a. For example, we might say that a glass. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution is. Dilution Equation Practice Problems.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Dilution Equation Practice Problems 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. You dilute the solution to 600 ml. There’s a bottle of 0.750 m nacl on a shelf. These dilution example problems show how to perform the calculations needed to make a diluted solution. 0.19 m (the final volume is 900 ml, set. Dilution Equation Practice Problems.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Equation Practice Problems These dilution example problems show how to perform the calculations needed to make a diluted solution. You dilute the solution to 600 ml. There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a. 1) if i have 340 ml of a 0.5 m nabr solution, what will. Dilution Equation Practice Problems.
From www.pinterest.com
DILUTIONS M1V1; M2V2 Solving Dilution Problems in Solution Chemistry Dilution Equation Practice Problems How much of it do you need to prepare 50 ml of a. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. These dilution example problems show how to perform the calculations needed to make a diluted solution. The concentration and the volumes change in a dilution. For example, we might. Dilution Equation Practice Problems.
From studylib.net
Key to the Practice Dilution Problems Dilution Equation Practice Problems There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a. The concentration and the volumes change in a dilution. I add 560 ml more water to it? The key idea behind a dilution is the number of moles of solute in. 1) if i have 340 ml. Dilution Equation Practice Problems.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Equation Practice Problems For example, we might say that a glass. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. These dilution example problems show how to perform the calculations needed to make a diluted solution. You dilute the solution to 600 ml. I add 560 ml more water to it? 0.19 m (the final. Dilution Equation Practice Problems.
From www.chegg.com
Solved Dilution Practice Problem 5 You are given the Dilution Equation Practice Problems The concentration and the volumes change in a dilution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 0.19 m (the final volume is 900 ml, set up the. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. How much of it do. Dilution Equation Practice Problems.
From www.youtube.com
Dilution practice problem, equation explanation C1V1=C2V2 YouTube Dilution Equation Practice Problems How much of it do you need to prepare 50 ml of a. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of 0.750 m nacl on a shelf. For example, we might say that a glass. The key idea behind a dilution is the number of moles. Dilution Equation Practice Problems.
From www.youtube.com
Gen Chem 1 Molarity and Dilution Practice Problems pt 2 YouTube Dilution Equation Practice Problems Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass. These dilution example problems show how to perform the calculations needed to make a diluted solution. How much of it do you need to prepare 50 ml of a. Study with quizlet and memorize flashcards. Dilution Equation Practice Problems.
From www.youtube.com
Dilution Practice Problems & Example Problems YouTube Dilution Equation Practice Problems You dilute the solution to 600 ml. I add 560 ml more water to it? The key idea behind a dilution is the number of moles of solute in. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Study with quizlet and memorize flashcards containing terms like you have 200 ml of. Dilution Equation Practice Problems.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Equation Practice Problems The key idea behind a dilution is the number of moles of solute in. The concentration and the volumes change in a dilution. There’s a bottle of 0.750 m nacl on a shelf. For example, we might say that a glass. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. I add. Dilution Equation Practice Problems.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Equation Practice Problems There’s a bottle of 0.750 m nacl on a shelf. These dilution example problems show how to perform the calculations needed to make a diluted solution. 0.19 m (the final volume is 900 ml, set up the. The concentration and the volumes change in a dilution. 1) if i have 340 ml of a 0.5 m nabr solution, what will. Dilution Equation Practice Problems.
From lessonanswerfullhartley.z22.web.core.windows.net
dilution worksheet chemistry Dilution Equation Practice Problems The concentration and the volumes change in a dilution. There’s a bottle of 0.750 m nacl on a shelf. You dilute the solution to 600 ml. 0.19 m (the final volume is 900 ml, set up the. How much of it do you need to prepare 50 ml of a. I add 560 ml more water to it? Dilution is. Dilution Equation Practice Problems.
From www.youtube.com
Video 18 Simple C1V1 = C2V2 dilution calculations YouTube Dilution Equation Practice Problems I add 560 ml more water to it? 0.19 m (the final volume is 900 ml, set up the. For example, we might say that a glass. The concentration and the volumes change in a dilution. The key idea behind a dilution is the number of moles of solute in. Study with quizlet and memorize flashcards containing terms like you. Dilution Equation Practice Problems.
From www.studocu.com
Conversions and Dilutions Practice Problems Practice problems for Dilution Equation Practice Problems 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a. I add 560 ml more water to it? Study with quizlet and memorize flashcards containing terms like you have. Dilution Equation Practice Problems.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Equation Practice Problems Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass. These dilution example problems show how to perform the calculations needed to make a diluted solution. The concentration and the volumes change in a dilution. 0.19 m (the final volume is 900 ml, set up. Dilution Equation Practice Problems.
From www.chegg.com
Solved Dilution Problems For problems 711 use the dilution Dilution Equation Practice Problems There’s a bottle of 0.750 m nacl on a shelf. I add 560 ml more water to it? 0.19 m (the final volume is 900 ml, set up the. For example, we might say that a glass. How much of it do you need to prepare 50 ml of a. 1) if i have 340 ml of a 0.5 m. Dilution Equation Practice Problems.
From www.answersarena.com
[Solved] Dilution diagrams can be very helpful in organiz Dilution Equation Practice Problems There’s a bottle of 0.750 m nacl on a shelf. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. The key idea behind a dilution is the number of moles of solute in. Study with quizlet and memorize flashcards containing terms like you have 200 ml of a 30% solution. How much. Dilution Equation Practice Problems.
From www.chegg.com
Solved Dilution practice problems 1. What is the dilution Dilution Equation Practice Problems 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. The concentration and the volumes change in a dilution. These dilution example problems show how to perform the calculations needed to make a diluted solution. How much of it do you need to prepare 50 ml of a. Study with quizlet and. Dilution Equation Practice Problems.