Which Atom In Nocl Is Closest To The Negative Side . As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. For nocl, what atom is closest to negative side? In its most stable state, nitrogen acts as the central atom and forms a double bond with. In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. For nocl, what atom is closest to negative side? In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. There are 2 steps to solve this one. The polarity of an atom. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. The negative charge of electrons balances the positive charge of protons in an atom. The electrons symmetrically distributed around the nucleus leave no negative.
from www.numerade.com
The polarity of an atom. For nocl, what atom is closest to negative side? In its most stable state, nitrogen acts as the central atom and forms a double bond with. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. The negative charge of electrons balances the positive charge of protons in an atom. For nocl, what atom is closest to negative side? In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,.
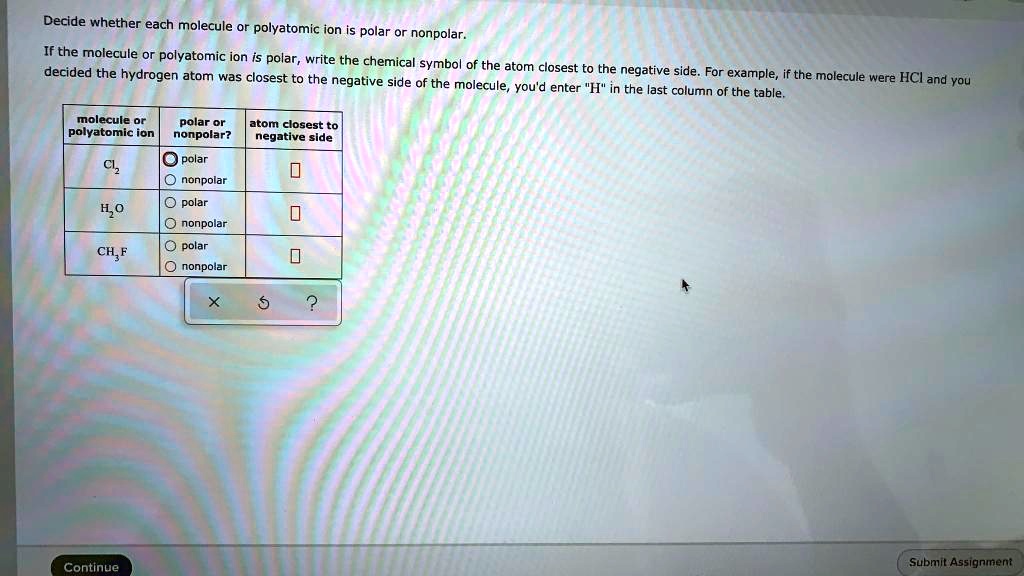
SOLVED Decide whether each molecule or polyatomic ion is polar or
Which Atom In Nocl Is Closest To The Negative Side For nocl, what atom is closest to negative side? Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. For nocl, what atom is closest to negative side? For nocl, what atom is closest to negative side? As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. The electrons symmetrically distributed around the nucleus leave no negative. There are 2 steps to solve this one. In its most stable state, nitrogen acts as the central atom and forms a double bond with. In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. The negative charge of electrons balances the positive charge of protons in an atom. The polarity of an atom.
From www.youtube.com
NOCl Lewis Structure How to Draw the Lewis Structure for NOCl YouTube Which Atom In Nocl Is Closest To The Negative Side The polarity of an atom. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. For nocl, what atom is closest to negative side? There are 2 steps to solve this one. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. The negative charge of electrons. Which Atom In Nocl Is Closest To The Negative Side.
From lucia-has-beasley.blogspot.com
F2 Polar or Nonpolar Atom Closest to Negative Side LuciahasBeasley Which Atom In Nocl Is Closest To The Negative Side There are 2 steps to solve this one. The electrons symmetrically distributed around the nucleus leave no negative. In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. The polarity of an atom. The negative charge of electrons balances the positive charge of protons in an atom. For nocl, what atom is. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved Decide whether each molecule or polyatomic ion is Which Atom In Nocl Is Closest To The Negative Side The negative charge of electrons balances the positive charge of protons in an atom. The electrons symmetrically distributed around the nucleus leave no negative. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. For nocl, what atom is closest to negative side? The polarity of an atom. There are 2 steps to solve this one. For. Which Atom In Nocl Is Closest To The Negative Side.
From nathenkruwbaker.blogspot.com
Clf Polar or Nonpolar Atom Closest to Negative Side NathenkruwBaker Which Atom In Nocl Is Closest To The Negative Side In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. For nocl, what atom is closest to negative side? The negative charge of electrons balances the positive charge of protons in. Which Atom In Nocl Is Closest To The Negative Side.
From www.numerade.com
SOLVED Decide whether each molecule or polyatomic ion is polar or Which Atom In Nocl Is Closest To The Negative Side In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. The electrons symmetrically distributed around the nucleus leave no negative. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. As mentioned in section 4.7, because the electrons in. Which Atom In Nocl Is Closest To The Negative Side.
From www.coursehero.com
[Solved] Decide whether each molecule or polyatomic ion is polar or Which Atom In Nocl Is Closest To The Negative Side There are 2 steps to solve this one. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. The negative charge of electrons balances the positive charge of protons. Which Atom In Nocl Is Closest To The Negative Side.
From www.numerade.com
SOLVED Text Decide whether each molecule or polyatomic ion is polar Which Atom In Nocl Is Closest To The Negative Side Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. In the nocl lewis structure nitrogen (n) is the least electronegative atom and. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved Decide whether each molecule or polyatomic ion is Which Atom In Nocl Is Closest To The Negative Side For nocl, what atom is closest to negative side? In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. The negative charge of electrons balances the positive charge of protons in an atom. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved If the molecule or polyatomic ion is polar, write the Which Atom In Nocl Is Closest To The Negative Side Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. The electrons symmetrically distributed around the nucleus leave no negative. The polarity of. Which Atom In Nocl Is Closest To The Negative Side.
From www.catmint.com
ch3cl atom closest to negative side Which Atom In Nocl Is Closest To The Negative Side Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. The electrons symmetrically distributed around the nucleus leave no negative. For nocl, what atom is closest to negative side? As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved Decide whether each molecule or polyatomic ion is Which Atom In Nocl Is Closest To The Negative Side As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. The negative charge of electrons balances the positive charge of protons in. Which Atom In Nocl Is Closest To The Negative Side.
From www.numerade.com
SOLVED Decide whether each molecule or polyatomic ion is polar or Which Atom In Nocl Is Closest To The Negative Side For nocl, what atom is closest to negative side? In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. The negative charge of electrons balances the positive charge of protons in an. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved Decide whether each molecule or polyatomic ion is Which Atom In Nocl Is Closest To The Negative Side As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. The negative charge of electrons balances the positive charge of protons in an atom. For nocl, what atom is closest to negative side? The electrons symmetrically distributed around the nucleus leave no negative.. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved Decide whether each molecule or polyatomic ion is Which Atom In Nocl Is Closest To The Negative Side For nocl, what atom is closest to negative side? For nocl, what atom is closest to negative side? Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule. Which Atom In Nocl Is Closest To The Negative Side.
From jeopardylabs.com
Chemical Bonding and Nomenclature Jeopardy Template Which Atom In Nocl Is Closest To The Negative Side The negative charge of electrons balances the positive charge of protons in an atom. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved Decide whether each molecule or polyatomic ion is Which Atom In Nocl Is Closest To The Negative Side As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. There are 2 steps to solve this one. The polarity of an. Which Atom In Nocl Is Closest To The Negative Side.
From lucia-has-beasley.blogspot.com
F2 Polar or Nonpolar Atom Closest to Negative Side LuciahasBeasley Which Atom In Nocl Is Closest To The Negative Side Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. In its most stable state, nitrogen acts as the. Which Atom In Nocl Is Closest To The Negative Side.
From www.numerade.com
SOLVED Decide whether each molecule or polyatomic ion is polar or Which Atom In Nocl Is Closest To The Negative Side The polarity of an atom. In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. For nocl, what atom. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved hydrogen atom was closest to the negative molecule or Which Atom In Nocl Is Closest To The Negative Side In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. In the nocl lewis structure nitrogen (n) is the. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved Decide whether each molecule or polyatomic ion is If Which Atom In Nocl Is Closest To The Negative Side In its most stable state, nitrogen acts as the central atom and forms a double bond with. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. The negative charge. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved Decide whether each molecule or polyatomic ion is Which Atom In Nocl Is Closest To The Negative Side There are 2 steps to solve this one. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. For nocl, what atom is closest to negative side? Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. As mentioned in section 4.7, because the electrons in. Which Atom In Nocl Is Closest To The Negative Side.
From www.numerade.com
SOLVED molecule or polar or atom closest to polyatomic ion nonpolar Which Atom In Nocl Is Closest To The Negative Side The electrons symmetrically distributed around the nucleus leave no negative. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. The polarity of an atom. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. In its most stable. Which Atom In Nocl Is Closest To The Negative Side.
From www.coursehero.com
[Solved] Decide whether each molecule or polyatomic ion is polar or Which Atom In Nocl Is Closest To The Negative Side For nocl, what atom is closest to negative side? As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. For nocl, what atom is closest to negative side? In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴. Which Atom In Nocl Is Closest To The Negative Side.
From www.numerade.com
SOLVED Decide whether each molecule polyatomic ion polar or nonpolar Which Atom In Nocl Is Closest To The Negative Side The electrons symmetrically distributed around the nucleus leave no negative. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. For nocl, what atom is closest to negative side? There are 2 steps to solve this one. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of. Which Atom In Nocl Is Closest To The Negative Side.
From www.vrogue.co
Ch4 Polar Or Nonpolar Covalent Bond Ppt The Chemistry vrogue.co Which Atom In Nocl Is Closest To The Negative Side In its most stable state, nitrogen acts as the central atom and forms a double bond with. The polarity of an atom. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. The electrons symmetrically distributed around the nucleus leave no negative. For nocl, what atom is closest to. Which Atom In Nocl Is Closest To The Negative Side.
From www.coursehero.com
Decide whether each molecule or polyatomic ion is polar or... Course Hero Which Atom In Nocl Is Closest To The Negative Side For nocl, what atom is closest to negative side? Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. In the nocl lewis. Which Atom In Nocl Is Closest To The Negative Side.
From www.chegg.com
Solved Decide whether each molecule or polyatomic ion is Which Atom In Nocl Is Closest To The Negative Side The polarity of an atom. For nocl, what atom is closest to negative side? The electrons symmetrically distributed around the nucleus leave no negative. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. In its most stable state, nitrogen acts as the central atom and forms a double bond with. In nocl, a is nitrogen, we. Which Atom In Nocl Is Closest To The Negative Side.
From coretanindah-indah.blogspot.com
Ch4 Polar Or Nonpolar Atom Closest To Negative Side Burning Methane Which Atom In Nocl Is Closest To The Negative Side The electrons symmetrically distributed around the nucleus leave no negative. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. The negative charge of electrons balances the positive charge of protons in an atom. In its most stable state, nitrogen acts as the central atom and forms a double. Which Atom In Nocl Is Closest To The Negative Side.
From www.vrogue.co
Ch4 Polar Or Nonpolar Atom Closest To Negative Side I vrogue.co Which Atom In Nocl Is Closest To The Negative Side In its most stable state, nitrogen acts as the central atom and forms a double bond with. For nocl, what atom is closest to negative side? Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. The negative charge of electrons balances the positive charge of protons in an atom.. Which Atom In Nocl Is Closest To The Negative Side.
From marcelogroharmon.blogspot.com
Ccl4 Polar or Nonpolar Atom Closest to Negative Side MarcelogroHarmon Which Atom In Nocl Is Closest To The Negative Side There are 2 steps to solve this one. In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. Here, a = central atom, x = surrounding atoms, and e = lone pair of electrons around the central atom. For nocl, what atom is closest to negative side? In its. Which Atom In Nocl Is Closest To The Negative Side.
From www.coursehero.com
[Solved] Decide whether each molecule or polyatomic ion is polar or Which Atom In Nocl Is Closest To The Negative Side In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. The negative charge of electrons balances the positive charge of protons in an atom. In its most stable state, nitrogen acts as the central atom and forms a double bond with. For nocl, what atom is closest to negative. Which Atom In Nocl Is Closest To The Negative Side.
From www.transtutors.com
(Solved) Molecule Or Polyatomic Ion Polar Or Nonpolar? Atom Closest Which Atom In Nocl Is Closest To The Negative Side In the nocl lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine. Which Atom In Nocl Is Closest To The Negative Side.
From www.coursehero.com
[Solved] Deci w whether each molecule or polyatomic ion is polar or Which Atom In Nocl Is Closest To The Negative Side There are 2 steps to solve this one. As mentioned in section 4.7, because the electrons in the bond are nearer to the f atom, this side of the molecule takes on a partial negative. The polarity of an atom. For nocl, what atom is closest to negative side? In its most stable state, nitrogen acts as the central atom. Which Atom In Nocl Is Closest To The Negative Side.
From en.wikipedia.org
Nitrosyl chloride Wikipedia Which Atom In Nocl Is Closest To The Negative Side The polarity of an atom. Nocl consists of one nitrogen atom, one oxygen atom, and one chlorine atom. In nocl, a is nitrogen, we have one o and one cl around nitrogen, ∴ ‘n’ = 2,. For nocl, what atom is closest to negative side? The negative charge of electrons balances the positive charge of protons in an atom. In. Which Atom In Nocl Is Closest To The Negative Side.
From ar.inspiredpencil.com
Nocl Molecular Geometry Which Atom In Nocl Is Closest To The Negative Side The polarity of an atom. In its most stable state, nitrogen acts as the central atom and forms a double bond with. The negative charge of electrons balances the positive charge of protons in an atom. For nocl, what atom is closest to negative side? The electrons symmetrically distributed around the nucleus leave no negative. Nocl consists of one nitrogen. Which Atom In Nocl Is Closest To The Negative Side.