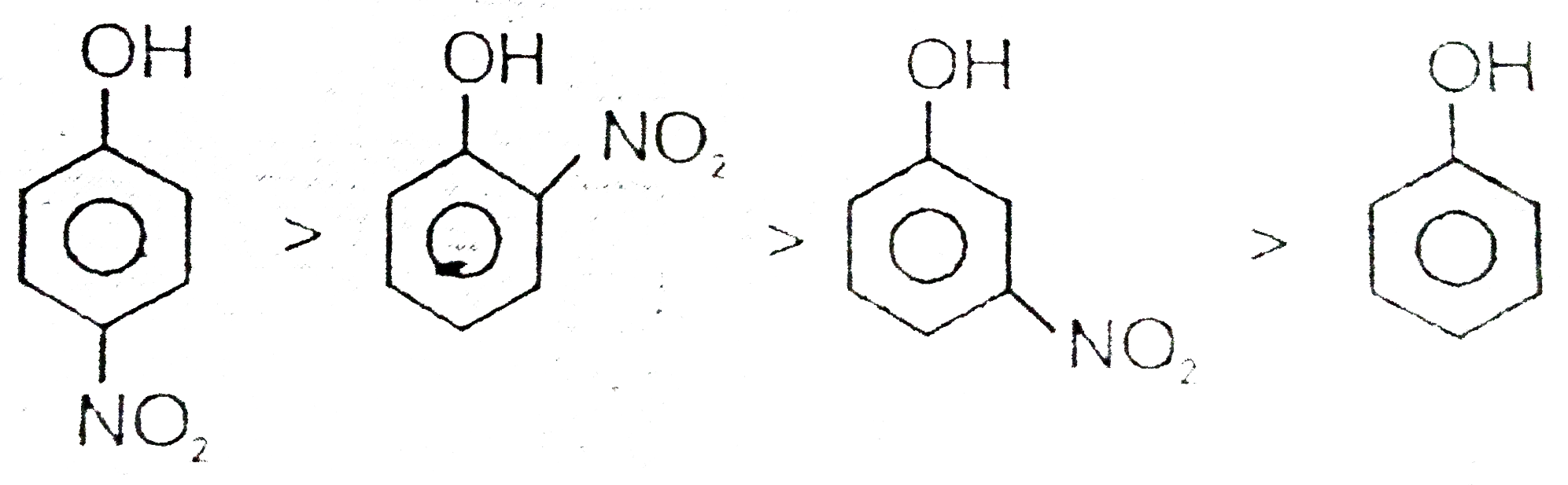
Which Is More Acidic Phenol Or Alcohol . Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Both alcohols and phenols are capable of acting as weakly acidic species; Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. Phenols are more acidic than alcohol. Phenols react with active metals like sodium, and potassium to form phenoxide. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. However, phenol is sufficiently acidic for. Explain, in terms of inductive. This reaction of phenol with metals indicates its acidic nature. When deprotonated, their conjugate bases are both. Explain why phenols are more acidic than alcohols. Explain the difference in acidity between two given alcohols or phenols.
from www.doubtnut.com
Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Both alcohols and phenols are capable of acting as weakly acidic species; This reaction of phenol with metals indicates its acidic nature. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. When deprotonated, their conjugate bases are both. However, phenol is sufficiently acidic for. Explain the difference in acidity between two given alcohols or phenols. Phenols are more acidic than alcohol. Explain, in terms of inductive. Phenols react with active metals like sodium, and potassium to form phenoxide.
(a) Why acidic nature of alcohol and phenol increase with electr
Which Is More Acidic Phenol Or Alcohol This reaction of phenol with metals indicates its acidic nature. Explain, in terms of inductive. However, phenol is sufficiently acidic for. Explain why phenols are more acidic than alcohols. When deprotonated, their conjugate bases are both. Phenols react with active metals like sodium, and potassium to form phenoxide. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. This reaction of phenol with metals indicates its acidic nature. Both alcohols and phenols are capable of acting as weakly acidic species; Phenols are more acidic than alcohol. Explain the difference in acidity between two given alcohols or phenols. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Delocalization of the negative charge over the ortho and para positions of the aromatic ring.
From www.youtube.com
Why is phenol more acidic than ethanol ? YouTube Which Is More Acidic Phenol Or Alcohol However, phenol is sufficiently acidic for. Explain, in terms of inductive. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
The correct order of relative acidic strength of phenol, ethyl alcohol and water is YouTube Which Is More Acidic Phenol Or Alcohol Phenols are more acidic than alcohol. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Phenols react with active metals like sodium, and potassium to form phenoxide. Both alcohols and phenols are capable of acting as weakly acidic species; Explain why phenols are more acidic than alcohols. Explain the difference in acidity between. Which Is More Acidic Phenol Or Alcohol.
From chemizi.blogspot.com
Why acetic acid is more acidic than phenol and Why phenol are more acidic than aliphatic alcohol Which Is More Acidic Phenol Or Alcohol Explain, in terms of inductive. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Explain the difference in acidity between two given alcohols or phenols. This reaction of phenol with metals indicates its acidic nature. Phenols are more. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
Phenol is More Acidic than Ethanol Alcohols, Phenols and Ethers Chemistry Class 12 YouTube Which Is More Acidic Phenol Or Alcohol Explain the difference in acidity between two given alcohols or phenols. Phenols are more acidic than alcohol. Explain, in terms of inductive. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Explain why phenols are more acidic than alcohols. Delocalization of the negative charge over the ortho and para positions of the aromatic. Which Is More Acidic Phenol Or Alcohol.
From slideplayer.com
Chapter 17 Alcohols and Phenols ppt download Which Is More Acidic Phenol Or Alcohol However, phenol is sufficiently acidic for. Explain, in terms of inductive. Phenols react with active metals like sodium, and potassium to form phenoxide. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain the difference in acidity between. Which Is More Acidic Phenol Or Alcohol.
From www.slideserve.com
PPT Alcohols PowerPoint Presentation, free download ID3982776 Which Is More Acidic Phenol Or Alcohol Phenols are more acidic than alcohol. This reaction of phenol with metals indicates its acidic nature. Explain, in terms of inductive. However, phenol is sufficiently acidic for. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. Alcohols are so weakly acidic that, for normal lab purposes,. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
Phenols are more acidic than alcohals.why YouTube Which Is More Acidic Phenol Or Alcohol Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. This reaction of phenol with metals indicates its acidic nature. Explain the difference in acidity between two given alcohols or phenols. Explain why phenols are more acidic than alcohols. Delocalization of the negative charge over the ortho. Which Is More Acidic Phenol Or Alcohol.
From leah4sci.com
Phenol Resonance and Acidity Organic Chemistry Video Which Is More Acidic Phenol Or Alcohol Phenols are more acidic than alcohol. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Explain, in terms of inductive. Explain the difference in acidity between two given alcohols or phenols. Phenols react with active metals like sodium, and potassium to form phenoxide. Phenol is more acidic than ethanol because in phenol lone. Which Is More Acidic Phenol Or Alcohol.
From www.doubtnut.com
(a) Why acidic nature of alcohol and phenol increase with electr Which Is More Acidic Phenol Or Alcohol Explain why phenols are more acidic than alcohols. This reaction of phenol with metals indicates its acidic nature. Both alcohols and phenols are capable of acting as weakly acidic species; However, phenol is sufficiently acidic for. Phenols react with active metals like sodium, and potassium to form phenoxide. Alcohols are so weakly acidic that, for normal lab purposes, their acidity. Which Is More Acidic Phenol Or Alcohol.
From www.doubtnut.com
Carboxylic acids are more acidic than phenols and alchols. Explin Which Is More Acidic Phenol Or Alcohol Explain the difference in acidity between two given alcohols or phenols. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. When deprotonated, their conjugate bases are both. Explain why phenols are more acidic than alcohols. Explain, in terms of inductive. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be. Which Is More Acidic Phenol Or Alcohol.
From www.imperialstudy.com
Alcohols Phenols and Ethers Notes for Class 12 Chemistry Imperial Study Which Is More Acidic Phenol Or Alcohol Both alcohols and phenols are capable of acting as weakly acidic species; Explain why phenols are more acidic than alcohols. Phenols react with active metals like sodium, and potassium to form phenoxide. Explain, in terms of inductive. This reaction of phenol with metals indicates its acidic nature. Delocalization of the negative charge over the ortho and para positions of the. Which Is More Acidic Phenol Or Alcohol.
From www.doubtnut.com
Why is phenol more acidic than ethanol Which Is More Acidic Phenol Or Alcohol When deprotonated, their conjugate bases are both. Phenols are more acidic than alcohol. Explain, in terms of inductive. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain the difference in acidity between two given alcohols or phenols.. Which Is More Acidic Phenol Or Alcohol.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID4524523 Which Is More Acidic Phenol Or Alcohol Explain the difference in acidity between two given alcohols or phenols. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. Explain, in terms of inductive. Phenols react with active metals like sodium, and potassium to form phenoxide. Delocalization of the negative charge over the ortho and. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
Why Phenol is more Acidic than Alcohol? A2 Chemistry (9701) YouTube Which Is More Acidic Phenol Or Alcohol Phenols are more acidic than alcohol. Explain the difference in acidity between two given alcohols or phenols. Explain, in terms of inductive. This reaction of phenol with metals indicates its acidic nature. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. However, phenol is sufficiently acidic for. Explain why phenols are more acidic. Which Is More Acidic Phenol Or Alcohol.
From www.slideserve.com
PPT Unit 6 Alcohols and Ethers PowerPoint Presentation, free download ID3209025 Which Is More Acidic Phenol Or Alcohol Explain, in terms of inductive. This reaction of phenol with metals indicates its acidic nature. However, phenol is sufficiently acidic for. Phenols react with active metals like sodium, and potassium to form phenoxide. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenol is more acidic than ethanol because in phenol lone pair of. Which Is More Acidic Phenol Or Alcohol.
From www.slideserve.com
PPT Alcohols PowerPoint Presentation, free download ID4396111 Which Is More Acidic Phenol Or Alcohol Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain the difference in acidity between two given alcohols or phenols. Alcohols are so weakly acidic that, for normal lab purposes, their. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
15) Acidic nature of phenol Why Phenol is more acidic than Alcohol class12 organic chemistry Which Is More Acidic Phenol Or Alcohol This reaction of phenol with metals indicates its acidic nature. However, phenol is sufficiently acidic for. Both alcohols and phenols are capable of acting as weakly acidic species; Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
14) Acidic nature of Alcohols why Water is more acidic than Alcohols Alcohol phenol ethers Which Is More Acidic Phenol Or Alcohol However, phenol is sufficiently acidic for. This reaction of phenol with metals indicates its acidic nature. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. When deprotonated, their conjugate bases are both. Explain the difference in acidity between two given alcohols or phenols. Phenols are more. Which Is More Acidic Phenol Or Alcohol.
From www.learnpick.in
Which one is more acidic phenol or acetic acid? Find 8 Answers & Solutions LearnPick Resources Which Is More Acidic Phenol Or Alcohol When deprotonated, their conjugate bases are both. However, phenol is sufficiently acidic for. Explain why phenols are more acidic than alcohols. This reaction of phenol with metals indicates its acidic nature. Explain, in terms of inductive. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Phenols are more acidic than alcohol. Phenol is. Which Is More Acidic Phenol Or Alcohol.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in detail. Which Is More Acidic Phenol Or Alcohol Explain, in terms of inductive. When deprotonated, their conjugate bases are both. Phenols react with active metals like sodium, and potassium to form phenoxide. Phenols are more acidic than alcohol. Both alcohols and phenols are capable of acting as weakly acidic species; Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Explain why. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
why phenol is more acidic than alcohol in hindi phenol Alcohol TPLive Maths YouTube Which Is More Acidic Phenol Or Alcohol Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. This reaction of phenol with metals indicates its acidic nature. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. However, phenol is sufficiently acidic for. Delocalization of the negative charge. Which Is More Acidic Phenol Or Alcohol.
From pediaa.com
Difference Between Alcohol and Phenol Definition, Structure, Use Which Is More Acidic Phenol Or Alcohol However, phenol is sufficiently acidic for. Phenols are more acidic than alcohol. When deprotonated, their conjugate bases are both. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain, in terms. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
Alcohol, Phenol & Ether 8 Preparation of Phenol Acidic nature of phenol YouTube Which Is More Acidic Phenol Or Alcohol This reaction of phenol with metals indicates its acidic nature. Explain, in terms of inductive. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. Phenols are more acidic than alcohol. When deprotonated, their conjugate bases are both. Explain the difference in acidity between two given alcohols. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
Comment Carboxylic acid is More acidic than phenol and alcohol YouTube Which Is More Acidic Phenol Or Alcohol Explain why phenols are more acidic than alcohols. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. However, phenol is sufficiently acidic for. Explain the difference in acidity between two given alcohols or phenols. When deprotonated, their conjugate bases are both. Phenols react with active metals like sodium, and potassium to form phenoxide. Phenols. Which Is More Acidic Phenol Or Alcohol.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID4786846 Which Is More Acidic Phenol Or Alcohol When deprotonated, their conjugate bases are both. Explain the difference in acidity between two given alcohols or phenols. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain why phenols are more acidic than alcohols. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so. Which Is More Acidic Phenol Or Alcohol.
From www.numerade.com
SOLVEDCarboxylic acids are more acidic than phenol and alcohol because of (a) Formation of Which Is More Acidic Phenol Or Alcohol Explain, in terms of inductive. Explain why phenols are more acidic than alcohols. However, phenol is sufficiently acidic for. When deprotonated, their conjugate bases are both. Phenols react with active metals like sodium, and potassium to form phenoxide. This reaction of phenol with metals indicates its acidic nature. Explain the difference in acidity between two given alcohols or phenols. Both. Which Is More Acidic Phenol Or Alcohol.
From byjus.com
Phenol is having more acidic character than alcohol. Explain why? Which Is More Acidic Phenol Or Alcohol Explain the difference in acidity between two given alcohols or phenols. Phenols react with active metals like sodium, and potassium to form phenoxide. When deprotonated, their conjugate bases are both. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Phenols are more acidic than alcohol. However, phenol is sufficiently acidic for. Explain, in. Which Is More Acidic Phenol Or Alcohol.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and Sulfides PowerPoint Which Is More Acidic Phenol Or Alcohol Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. However, phenol is sufficiently acidic for. Phenols are more acidic than alcohol. Explain, in terms of inductive. Both alcohols and phenols are capable of acting as weakly acidic species; Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized. Which Is More Acidic Phenol Or Alcohol.
From www.youtube.com
Phenol is more acidic than alcohol...why?... YouTube Which Is More Acidic Phenol Or Alcohol Phenols react with active metals like sodium, and potassium to form phenoxide. Explain the difference in acidity between two given alcohols or phenols. Explain why phenols are more acidic than alcohols. However, phenol is sufficiently acidic for. When deprotonated, their conjugate bases are both. Phenols are more acidic than alcohol. Phenol is more acidic than ethanol because in phenol lone. Which Is More Acidic Phenol Or Alcohol.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Which Is More Acidic Phenol Or Alcohol Both alcohols and phenols are capable of acting as weakly acidic species; This reaction of phenol with metals indicates its acidic nature. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols are more acidic than alcohol. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Explain,. Which Is More Acidic Phenol Or Alcohol.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Which Is More Acidic Phenol Or Alcohol Explain why phenols are more acidic than alcohols. Both alcohols and phenols are capable of acting as weakly acidic species; Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain the difference in acidity between two given alcohols or phenols. Phenols react with active metals like sodium, and potassium to form phenoxide. When deprotonated,. Which Is More Acidic Phenol Or Alcohol.
From sciencebyjus.blogspot.com
Acidic Nature of Phenols? Why phenols are more acidic than alcohol Scientific Which Is More Acidic Phenol Or Alcohol Explain, in terms of inductive. When deprotonated, their conjugate bases are both. Explain the difference in acidity between two given alcohols or phenols. However, phenol is sufficiently acidic for. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Both alcohols and phenols are capable of acting as weakly acidic species; Phenols react with. Which Is More Acidic Phenol Or Alcohol.
From www.pdfprof.com
comparison of acidity of phenols and alcohols Which Is More Acidic Phenol Or Alcohol Explain, in terms of inductive. Phenols react with active metals like sodium, and potassium to form phenoxide. Both alcohols and phenols are capable of acting as weakly acidic species; Explain why phenols are more acidic than alcohols. When deprotonated, their conjugate bases are both. Explain the difference in acidity between two given alcohols or phenols. However, phenol is sufficiently acidic. Which Is More Acidic Phenol Or Alcohol.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in detail. Which Is More Acidic Phenol Or Alcohol Explain, in terms of inductive. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between o. However, phenol is sufficiently acidic for. When deprotonated, their conjugate bases are both. This reaction of. Which Is More Acidic Phenol Or Alcohol.
From www.slideserve.com
PPT Structures of Alcohols, Phenols, Thiols and Ethers PowerPoint Presentation ID6592973 Which Is More Acidic Phenol Or Alcohol Explain the difference in acidity between two given alcohols or phenols. This reaction of phenol with metals indicates its acidic nature. Explain, in terms of inductive. Phenols react with active metals like sodium, and potassium to form phenoxide. Phenols are more acidic than alcohol. However, phenol is sufficiently acidic for. Delocalization of the negative charge over the ortho and para. Which Is More Acidic Phenol Or Alcohol.