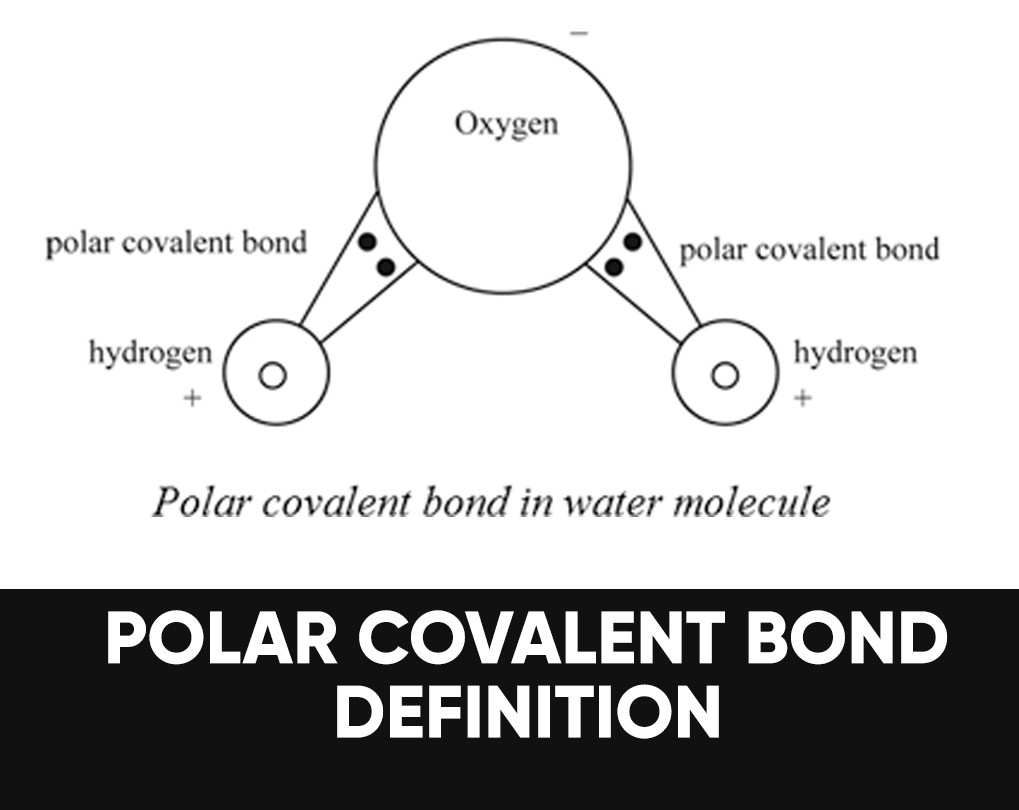
Is Acetone Polar Covalent . Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. The polarity of a covalent bond. There are different degrees of polarity, and acetone is less polar than water,. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Because of the carbonyl group, acetone is a somewhat polar molecule. Is acetone polar or nonpolar? As a result, the dipole moment of.
from www.orangatame.com
As a result, the dipole moment of. Because of the carbonyl group, acetone is a somewhat polar molecule. The polarity of a covalent bond. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. There are different degrees of polarity, and acetone is less polar than water,. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Is acetone polar or nonpolar? A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom.
Polar Covalent Bond Definition, Properties, Examples
Is Acetone Polar Covalent A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Because of the carbonyl group, acetone is a somewhat polar molecule. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. As a result, the dipole moment of. Polarity is the phenomenon where charges are separated to form an electric dipole moment. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Is acetone polar or nonpolar? The polarity of a covalent bond. There are different degrees of polarity, and acetone is less polar than water,. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom.
From physvids.com
Polar Covalent Bonds Clearly Explained for Easy Learning Is Acetone Polar Covalent The polarity of a covalent bond. Because of the carbonyl group, acetone is a somewhat polar molecule. There are different degrees of polarity, and acetone is less polar than water,. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. A bond in which the electronegativity. Is Acetone Polar Covalent.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone Polar Covalent Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. There are different degrees of polarity, and acetone is less polar than water,. Polarity is the phenomenon where charges are separated to form an electric dipole moment. The molecules that compose acetone do have nonpolar covalent. Is Acetone Polar Covalent.
From animalia-life.club
Acetone Lewis Structure With Polarity Is Acetone Polar Covalent As a result, the dipole moment of. Is acetone polar or nonpolar? The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. There are. Is Acetone Polar Covalent.
From www.sliderbase.com
Polar Covalent Bonds Acids and Bases Presentation Chemistry Is Acetone Polar Covalent The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. Because of the carbonyl group, acetone is a somewhat polar molecule. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom.. Is Acetone Polar Covalent.
From byjus.com
Giving suitable examples, describe the formation of a polar covalent bond. Is Acetone Polar Covalent The polarity of a covalent bond. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Polarity is the phenomenon where charges are separated to form an electric dipole moment. As a result, the dipole moment of. The molecules that compose acetone do have nonpolar covalent bonds within their. Is Acetone Polar Covalent.
From www.windward.solutions
How does a polar bond differ from a covalent bond Is Acetone Polar Covalent Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Polarity is the phenomenon where charges are separated to form an electric dipole moment. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. As. Is Acetone Polar Covalent.
From www.chegg.com
Solved What happens to acetone when it is boiled? Select Is Acetone Polar Covalent There are different degrees of polarity, and acetone is less polar than water,. Because of the carbonyl group, acetone is a somewhat polar molecule. Is acetone polar or nonpolar? The polarity of a covalent bond. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. The. Is Acetone Polar Covalent.
From mungfali.com
What Is Polar Covalent Bond Is Acetone Polar Covalent The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Because of the carbonyl group, acetone is a somewhat polar molecule.. Is Acetone Polar Covalent.
From courses.lumenlearning.com
Reading Covalent Bonds Biology I Is Acetone Polar Covalent A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Because of the carbonyl group, acetone is a somewhat polar molecule. There are different degrees of polarity, and acetone is less polar than water,. The polarity of a covalent bond. Acetone is a polar substance because of polarity in. Is Acetone Polar Covalent.
From www.slideserve.com
PPT 4.2 Covalent Bonding PowerPoint Presentation ID432101 Is Acetone Polar Covalent Because of the carbonyl group, acetone is a somewhat polar molecule. Polarity is the phenomenon where charges are separated to form an electric dipole moment. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Acetone is a polar substance because of polarity in the carbonyl group due to. Is Acetone Polar Covalent.
From brainly.com
indicate polar bonds for acetone. draw a vector representing the Is Acetone Polar Covalent As a result, the dipole moment of. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Is acetone polar or nonpolar? Polarity is the phenomenon where charges are separated to form an electric dipole moment. Because of the carbonyl group, acetone is a somewhat polar molecule. The polarity. Is Acetone Polar Covalent.
From www.sliderbase.com
Polar Covalent Bonds Acids and Bases Presentation Chemistry Is Acetone Polar Covalent As a result, the dipole moment of. Polarity is the phenomenon where charges are separated to form an electric dipole moment. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. There are different degrees of polarity, and acetone is less polar than water,. Because. Is Acetone Polar Covalent.
From www.youtube.com
POLAR COVALENT BOND CONCEPTUALLY EXPLAINED WITH EXAMPLES NI Concepts Is Acetone Polar Covalent Because of the carbonyl group, acetone is a somewhat polar molecule. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. The polarity of a covalent bond. Is acetone polar or nonpolar? A bond in which the electronegativity difference between the atoms is between 0.5. Is Acetone Polar Covalent.
From www.orangatame.com
Polar Covalent Bond Definition, Properties, Examples Is Acetone Polar Covalent Is acetone polar or nonpolar? A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. As a result, the dipole moment of. Because of the. Is Acetone Polar Covalent.
From www.slideserve.com
PPT Bond Polarity PowerPoint Presentation, free download ID6597592 Is Acetone Polar Covalent Polarity is the phenomenon where charges are separated to form an electric dipole moment. The polarity of a covalent bond. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. There are different degrees of polarity, and acetone is less polar than water,. A bond. Is Acetone Polar Covalent.
From www.techno-science.net
Acétone Définition et Explications Is Acetone Polar Covalent Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. The polarity of a covalent bond. As a result, the dipole moment of. There are different degrees of polarity, and acetone is less polar than water,. The molecules that compose acetone do have nonpolar covalent bonds. Is Acetone Polar Covalent.
From www.britannica.com
covalent bond Definition, Properties, Examples, & Facts Britannica Is Acetone Polar Covalent Is acetone polar or nonpolar? Polarity is the phenomenon where charges are separated to form an electric dipole moment. Because of the carbonyl group, acetone is a somewhat polar molecule. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. As a result, the dipole. Is Acetone Polar Covalent.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone Polar Covalent There are different degrees of polarity, and acetone is less polar than water,. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Polarity is the phenomenon where charges are separated to form an electric dipole moment. As a result, the dipole moment of. The molecules that compose acetone. Is Acetone Polar Covalent.
From www.thebigpicturemovie.com
Polar Covalent Bond Definition and Examples in Chemistry Is Acetone Polar Covalent Polarity is the phenomenon where charges are separated to form an electric dipole moment. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond.. Is Acetone Polar Covalent.
From www.orangatame.com
Polar Covalent Bond Definition, Properties, Examples Is Acetone Polar Covalent Is acetone polar or nonpolar? The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. The polarity of a covalent bond. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Acetone is a. Is Acetone Polar Covalent.
From www.slideserve.com
PPT Bond Polarity and Molecules PowerPoint Presentation, free Is Acetone Polar Covalent There are different degrees of polarity, and acetone is less polar than water,. The polarity of a covalent bond. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. As a result, the dipole moment of. Because of the carbonyl group, acetone is a somewhat polar molecule. Is acetone. Is Acetone Polar Covalent.
From www.makethebrainhappy.com
MakeTheBrainHappy Is Acetone Polar or Nonpolar? Is Acetone Polar Covalent The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. There are different degrees of polarity, and acetone is less polar than water,. The. Is Acetone Polar Covalent.
From chemistrymolecule.blogspot.com
Chemistry Molecule Acetone Is Acetone Polar Covalent The polarity of a covalent bond. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. As a result, the dipole moment of. There are different degrees of polarity, and acetone is less polar than water,. Is acetone polar or nonpolar? Polarity is the phenomenon. Is Acetone Polar Covalent.
From www.youtube.com
Is C3H6O (Acetone) Polar or NonPolar? YouTube Is Acetone Polar Covalent As a result, the dipole moment of. The polarity of a covalent bond. Is acetone polar or nonpolar? Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Polarity is the phenomenon where charges are separated to form an electric dipole moment. Because of the carbonyl. Is Acetone Polar Covalent.
From www.youtube.com
Acetone Lewis Structure How to Draw the Lewis Structure for Acetone Is Acetone Polar Covalent Because of the carbonyl group, acetone is a somewhat polar molecule. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. There are different degrees of polarity, and acetone is less polar than water,. A bond in which the electronegativity difference between the atoms is. Is Acetone Polar Covalent.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Is Acetone Polar Covalent The polarity of a covalent bond. As a result, the dipole moment of. Because of the carbonyl group, acetone is a somewhat polar molecule. Is acetone polar or nonpolar? Polarity is the phenomenon where charges are separated to form an electric dipole moment. There are different degrees of polarity, and acetone is less polar than water,. Acetone is a polar. Is Acetone Polar Covalent.
From physvids.com
Polar Covalent Bonds Clearly Explained for Easy Learning Is Acetone Polar Covalent There are different degrees of polarity, and acetone is less polar than water,. The polarity of a covalent bond. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Because of the carbonyl group, acetone is a somewhat polar molecule. Polarity is the phenomenon where charges are separated to. Is Acetone Polar Covalent.
From www.nagwa.com
Question Video Determining Whether Some Common Simple Molecular Is Acetone Polar Covalent Because of the carbonyl group, acetone is a somewhat polar molecule. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. The polarity of a covalent bond. Is acetone polar or nonpolar? Polarity is the phenomenon where charges are separated to form an electric dipole moment.. Is Acetone Polar Covalent.
From www.chemistrylearner.com
Polar Covalent Bond Definition and Examples Is Acetone Polar Covalent A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The polarity of a covalent bond. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. Acetone is a polar substance because of polarity. Is Acetone Polar Covalent.
From www.gizmoplans.com
Is Acetone Polar? Properties, Uses, Molecular Structure Is Acetone Polar Covalent As a result, the dipole moment of. Is acetone polar or nonpolar? Polarity is the phenomenon where charges are separated to form an electric dipole moment. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Acetone is a polar substance because of polarity in the carbonyl group due. Is Acetone Polar Covalent.
From www.pinterest.co.kr
polar bond Covalent bonding, Polar covalent bonds, Organic chemistry Is Acetone Polar Covalent Polarity is the phenomenon where charges are separated to form an electric dipole moment. There are different degrees of polarity, and acetone is less polar than water,. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Is acetone polar or nonpolar? The molecules that compose acetone do have. Is Acetone Polar Covalent.
From www.gizmoplans.com
Is Acetone Polar? Properties, Uses, Molecular Structure Is Acetone Polar Covalent Is acetone polar or nonpolar? There are different degrees of polarity, and acetone is less polar than water,. Polarity is the phenomenon where charges are separated to form an electric dipole moment. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. Because of the. Is Acetone Polar Covalent.
From physvids.com
Polar Covalent Bonds Clearly Explained for Easy Learning Is Acetone Polar Covalent Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. There are different degrees of polarity, and acetone is less polar than water,. Because of the carbonyl group, acetone is a somewhat polar molecule. As a result, the dipole moment of. Is acetone polar or nonpolar?. Is Acetone Polar Covalent.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Is Acetone Polar Covalent The polarity of a covalent bond. Polarity is the phenomenon where charges are separated to form an electric dipole moment. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Because of the carbonyl group, acetone is a somewhat polar molecule. As a result, the dipole moment of. The. Is Acetone Polar Covalent.
From www.exampleslab.com
20 Examples of Covalent Bonds Examples Lab Is Acetone Polar Covalent Is acetone polar or nonpolar? There are different degrees of polarity, and acetone is less polar than water,. The polarity of a covalent bond. Because of the carbonyl group, acetone is a somewhat polar molecule. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds.. Is Acetone Polar Covalent.