What Does A Buffer Do To An Acid . What is an acidic buffer? This characteristic makes buffers important in biological and. A ph buffer is a solution which resists ph change when acid or base are added to the solution. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An acidic buffer solution is. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. Firstly, we need to define what is a ph buffer. What is a buffer solution? A buffer (or buffered) solution is one that resists a.
from www.youtube.com
What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. An acidic buffer solution is. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. Firstly, we need to define what is a ph buffer. What is an acidic buffer? This characteristic makes buffers important in biological and. The mechanism involves a buffer, a solution that resists dramatic changes in ph. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)).
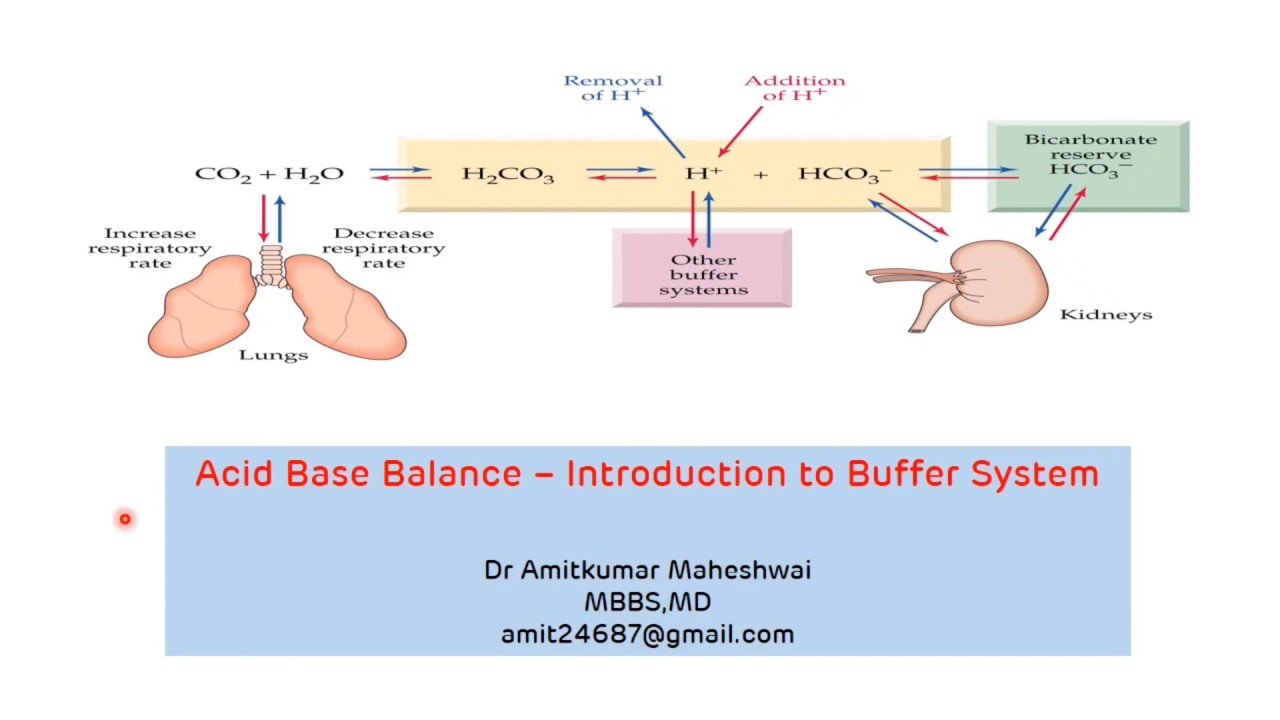
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers in
What Does A Buffer Do To An Acid If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: It is able to neutralize small amounts of added acid or base, thus maintaining the ph of. What is an acidic buffer? Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer (or buffered) solution is one that resists a. This characteristic makes buffers important in biological and. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. An acidic buffer solution is. What is a buffer solution? Firstly, we need to define what is a ph buffer. A ph buffer is a solution which resists ph change when acid or base are added to the solution. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download ID2119729 What Does A Buffer Do To An Acid Firstly, we need to define what is a ph buffer. A buffer (or buffered) solution is one that resists a. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). An acidic buffer solution is.. What Does A Buffer Do To An Acid.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination For Biologists What Does A Buffer Do To An Acid A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. What is a buffer solution? If a strong acid is added to a buffer, the. What Does A Buffer Do To An Acid.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 What Does A Buffer Do To An Acid It is able to neutralize small amounts of added acid or base, thus maintaining the ph of. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. What Does A Buffer Do To An Acid.
From mavink.com
Acids Bases And Buffers What Does A Buffer Do To An Acid A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. What is a buffer solution? A ph buffer is a solution which resists ph change when acid or base are added to the solution. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. Buffer solutions resist a change. What Does A Buffer Do To An Acid.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Does A Buffer Do To An Acid What is an acidic buffer? A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The mechanism involves a buffer, a solution that resists dramatic changes in ph. What is a buffer solution? An acidic buffer solution is. Buffer solutions resist a change in ph when small amounts. What Does A Buffer Do To An Acid.
From klafzqach.blob.core.windows.net
What Does A Buffer Do In Chemistry at Lura Laughlin blog What Does A Buffer Do To An Acid An acidic buffer solution is. What is an acidic buffer? A ph buffer is a solution which resists ph change when acid or base are added to the solution. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer (or buffered) solution is one that. What Does A Buffer Do To An Acid.
From www.peoi.org
Chapter 12 Section G Buffers What Does A Buffer Do To An Acid What is an acidic buffer? A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. Firstly, we need to define what is a ph buffer. A buffer (or buffered) solution is one that resists a. This characteristic makes buffers important in biological and. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are. What Does A Buffer Do To An Acid.
From www.youtube.com
Calculating the acidsalt ratio in a buffer YouTube What Does A Buffer Do To An Acid A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. What is an acidic buffer? Firstly, we need to define what is a ph buffer. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? The mechanism involves a buffer, a solution that resists dramatic. What Does A Buffer Do To An Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504 What Does A Buffer Do To An Acid A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. What is a buffer solution? A ph buffer is a solution which resists ph change when acid or base are added to the solution. A buffer is a solution. What Does A Buffer Do To An Acid.
From klafzqach.blob.core.windows.net
What Does A Buffer Do In Chemistry at Lura Laughlin blog What Does A Buffer Do To An Acid A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. What is an acidic buffer? Firstly, we need to define what is a ph buffer. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which resists changes in ph when small quantities of an. What Does A Buffer Do To An Acid.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Does A Buffer Do To An Acid It is able to neutralize small amounts of added acid or base, thus maintaining the ph of. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. This characteristic makes buffers important in biological and. The mechanism involves a buffer, a solution that resists dramatic changes in. What Does A Buffer Do To An Acid.
From klaknjndj.blob.core.windows.net
How Do Buffers Work Chemistry at Felix Balch blog What Does A Buffer Do To An Acid This characteristic makes buffers important in biological and. The mechanism involves a buffer, a solution that resists dramatic changes in ph. An acidic buffer solution is. A ph buffer is a solution which resists ph change when acid or base are added to the solution. Buffer solutions resist a change in ph when small amounts of a strong acid or. What Does A Buffer Do To An Acid.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid with strong base, weak base What Does A Buffer Do To An Acid Firstly, we need to define what is a ph buffer. This characteristic makes buffers important in biological and. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A ph buffer is a solution. What Does A Buffer Do To An Acid.
From www.expii.com
Buffers — Definition & Overview Expii What Does A Buffer Do To An Acid It is able to neutralize small amounts of added acid or base, thus maintaining the ph of. A buffer (or buffered) solution is one that resists a. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: A buffer is a solution. What Does A Buffer Do To An Acid.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Does A Buffer Do To An Acid A buffer (or buffered) solution is one that resists a. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small amounts of added acid or base, thus maintaining the ph of. Buffer solutions resist a change in ph when small amounts. What Does A Buffer Do To An Acid.
From www.slideserve.com
PPT A solution that contains a weak acid/conjugate base mixture or weak base/conjugate acid What Does A Buffer Do To An Acid This characteristic makes buffers important in biological and. An acidic buffer solution is. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: A ph buffer is a solution which resists ph change when acid or base are added to the solution.. What Does A Buffer Do To An Acid.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers in What Does A Buffer Do To An Acid A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. What is a buffer solution? This characteristic makes buffers important in biological and. It is able to neutralize small amounts of added acid or base, thus maintaining the. What Does A Buffer Do To An Acid.
From klaknjndj.blob.core.windows.net
How Do Buffers Work Chemistry at Felix Balch blog What Does A Buffer Do To An Acid A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer (or buffered) solution is one that resists a. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid. What Does A Buffer Do To An Acid.
From www.slideshare.net
Ch 18 buffers What Does A Buffer Do To An Acid A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. An acidic buffer solution is. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: It is able to neutralize. What Does A Buffer Do To An Acid.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog What Does A Buffer Do To An Acid An acidic buffer solution is. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: Buffer solutions resist a change. What Does A Buffer Do To An Acid.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Does A Buffer Do To An Acid What is a buffer solution? What is an acidic buffer? Firstly, we need to define what is a ph buffer. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. If a strong acid is added to a buffer, the. What Does A Buffer Do To An Acid.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Weak Acids and Buffers What Does A Buffer Do To An Acid If a strong acid is added to a buffer, the weak base will react with the h + from the strong acid to form the weak acid ha: The mechanism involves a buffer, a solution that resists dramatic changes in ph. An acidic buffer solution is. A ph buffer is a solution which resists ph change when acid or base. What Does A Buffer Do To An Acid.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Does A Buffer Do To An Acid A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A ph buffer is a solution which resists ph change when acid or base are added to the solution. A buffer (or buffered) solution is. What Does A Buffer Do To An Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504 What Does A Buffer Do To An Acid What is a buffer solution? Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Firstly, we need to define what is a ph buffer.. What Does A Buffer Do To An Acid.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Does A Buffer Do To An Acid A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? Buffer solutions resist a change in ph when small amounts. What Does A Buffer Do To An Acid.
From courses.lumenlearning.com
Buffers Chemistry for Majors What Does A Buffer Do To An Acid A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). What is an acidic buffer? A. What Does A Buffer Do To An Acid.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download ID556590 What Does A Buffer Do To An Acid A buffer (or buffered) solution is one that resists a. A ph buffer is a solution which resists ph change when acid or base are added to the solution. What is a buffer solution? The mechanism involves a buffer, a solution that resists dramatic changes in ph. This characteristic makes buffers important in biological and. What is an acidic buffer?. What Does A Buffer Do To An Acid.
From www.brainkart.com
Buffers What Does A Buffer Do To An Acid An acidic buffer solution is. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). What is a buffer solution? Firstly, we need to define what is a ph buffer. A buffer solution is one which resists changes in ph when small quantities of an acid or an. What Does A Buffer Do To An Acid.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Does A Buffer Do To An Acid A ph buffer is a solution which resists ph change when acid or base are added to the solution. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Firstly, we need to define what is a ph buffer. What is an acidic buffer? A solution of. What Does A Buffer Do To An Acid.
From content.byui.edu
Acids, Bases, pH and Buffers What Does A Buffer Do To An Acid Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer (or buffered) solution is one that resists a. Firstly, we need to define. What Does A Buffer Do To An Acid.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass What Does A Buffer Do To An Acid What is an acidic buffer? Firstly, we need to define what is a ph buffer. A ph buffer is a solution which resists ph change when acid or base are added to the solution. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A solution of. What Does A Buffer Do To An Acid.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Types of Buffer What Does A Buffer Do To An Acid It is able to neutralize small amounts of added acid or base, thus maintaining the ph of. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). If a strong acid is added to a buffer, the weak base will react with the h + from the strong. What Does A Buffer Do To An Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504 What Does A Buffer Do To An Acid The mechanism involves a buffer, a solution that resists dramatic changes in ph. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Firstly, we need to define what is a ph buffer. A buffer (or buffered) solution is one. What Does A Buffer Do To An Acid.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free download ID4769309 What Does A Buffer Do To An Acid A solution of acetic acid (\(\ce{ch3cooh}\) and sodium. This characteristic makes buffers important in biological and. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A ph buffer is a solution which resists ph change when acid or base are added to the solution. A buffer (or buffered) solution is. What Does A Buffer Do To An Acid.
From www.youtube.com
The Change in pH with the Addition of a Strong Acid to a Buffer YouTube What Does A Buffer Do To An Acid What is an acidic buffer? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A buffer is a solution that can resist ph change. What Does A Buffer Do To An Acid.