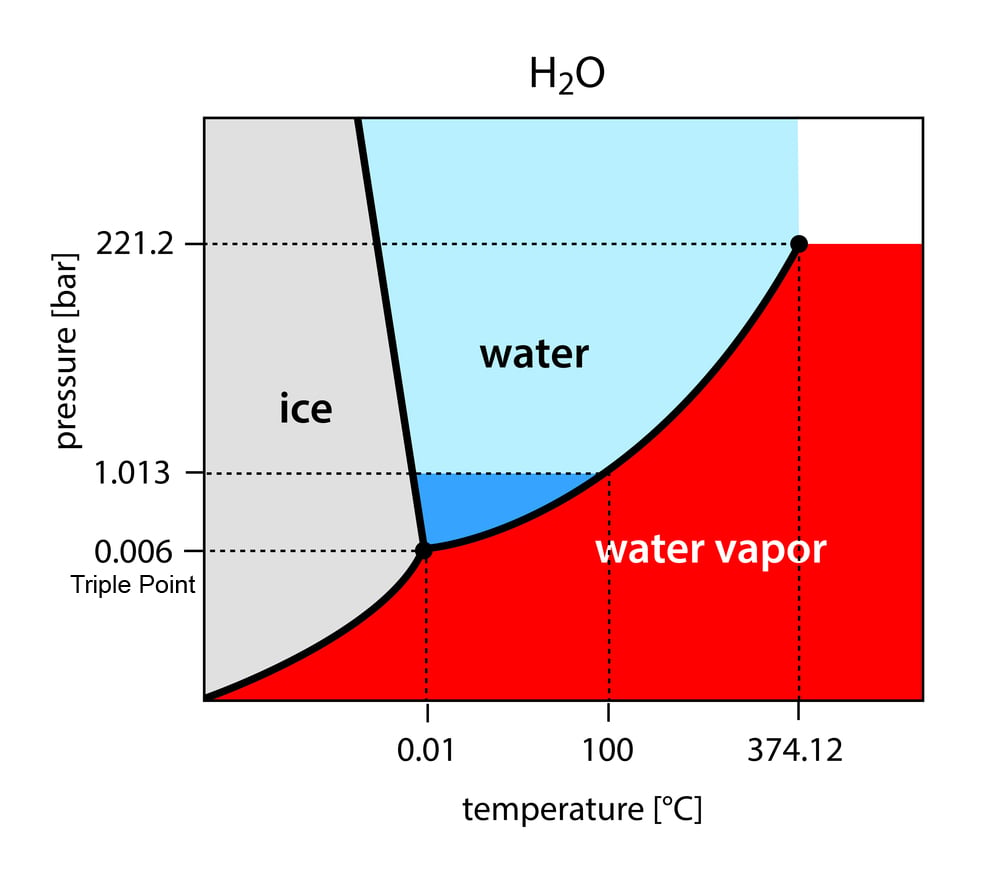
Solid And Liquid Phases Can Exist In Equilibrium Between Points . At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. Equilibrium between the liquid and gas phase will exist. These curves represent the relationships between phase. In figure \(\pageindex{1}\), the line that. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. The line that connects points a and b is the. Several distinct lines separate these phases. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. Crossing the line horizontally corresponds to melting or freezing. The solid and liquid phases are in equilibrium all along this line;
from www.scienceabc.com
In figure \(\pageindex{1}\), the line that. Crossing the line horizontally corresponds to melting or freezing. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. Several distinct lines separate these phases. These curves represent the relationships between phase. The line that connects points a and b is the. The solid and liquid phases are in equilibrium all along this line; The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. Equilibrium between the liquid and gas phase will exist.
Can All Elements Directly Transition From Solid To Gas? » ScienceABC
Solid And Liquid Phases Can Exist In Equilibrium Between Points At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. The line that connects points a and b is the. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. In figure \(\pageindex{1}\), the line that. The solid and liquid phases are in equilibrium all along this line; At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. Equilibrium between the liquid and gas phase will exist. These curves represent the relationships between phase. Crossing the line horizontally corresponds to melting or freezing. Several distinct lines separate these phases.
From unistudium.unipg.it
Phase Diagrams Solid And Liquid Phases Can Exist In Equilibrium Between Points Crossing the line horizontally corresponds to melting or freezing. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. Equilibrium between the liquid and gas phase will exist. The solid and liquid phases are in equilibrium all along this line; At the phase transition, such as the boiling. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.slideserve.com
PPT Chapter 11 States of Matter Liquids and Solids PowerPoint Solid And Liquid Phases Can Exist In Equilibrium Between Points The solid and liquid phases are in equilibrium all along this line; In figure \(\pageindex{1}\), the line that. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. Equilibrium between the liquid and gas phase will exist. Several distinct lines separate. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Chapter 11 Intermolecular Forces, Liquids, and Solids ppt download Solid And Liquid Phases Can Exist In Equilibrium Between Points These curves represent the relationships between phase. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. Equilibrium between the liquid and gas phase will exist. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,.. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Solid And Liquid Phases Can Exist In Equilibrium Between Points These curves represent the relationships between phase. Crossing the line horizontally corresponds to melting or freezing. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. The solid and liquid phases are in equilibrium all along this line; Several distinct lines. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Intermolecular Forces, Liquids, and Solids ppt download Solid And Liquid Phases Can Exist In Equilibrium Between Points The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. Several distinct lines separate these phases. At the phase transition, such as the boiling point between liquid and gas phases, the two states. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Solid And Liquid Phases Can Exist In Equilibrium Between Points Crossing the line horizontally corresponds to melting or freezing. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. Equilibrium between the liquid and gas phase will exist. In figure \(\pageindex{1}\), the line that. The line that connects points a and b is the. Several distinct lines separate these phases. At the phase transition, such. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry Solid And Liquid Phases Can Exist In Equilibrium Between Points Several distinct lines separate these phases. These curves represent the relationships between phase. The line that connects points a and b is the. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. The lines in a phase diagram correspond to. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Solid And Liquid Phases Can Exist In Equilibrium Between Points The solid and liquid phases are in equilibrium all along this line; These curves represent the relationships between phase. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. In figure \(\pageindex{1}\), the line that. The lines in a phase diagram. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.researchgate.net
Phase diagram and the phase equilibrium line 1 solid and liquid Solid And Liquid Phases Can Exist In Equilibrium Between Points Equilibrium between the liquid and gas phase will exist. Several distinct lines separate these phases. Crossing the line horizontally corresponds to melting or freezing. The solid and liquid phases are in equilibrium all along this line; The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. In figure \(\pageindex{1}\), the line that. These curves represent. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Unit 9 States of Matter. ppt download Solid And Liquid Phases Can Exist In Equilibrium Between Points The solid and liquid phases are in equilibrium all along this line; Several distinct lines separate these phases. The line that connects points a and b is the. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. In figure \(\pageindex{1}\),. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Equilibrium aned ppt download Solid And Liquid Phases Can Exist In Equilibrium Between Points Crossing the line horizontally corresponds to melting or freezing. Several distinct lines separate these phases. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. These curves represent the relationships between phase. Equilibrium between the liquid and gas phase will exist. At the phase transition, such as the. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.chemistrylearner.com
Phase Diagram Definition, Explanation, and Diagram Solid And Liquid Phases Can Exist In Equilibrium Between Points The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. The solid and liquid phases are in equilibrium all along this line; At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.numerade.com
SOLVED The figure below is the solidliquid phase diagram for the Solid And Liquid Phases Can Exist In Equilibrium Between Points Crossing the line horizontally corresponds to melting or freezing. The solid and liquid phases are in equilibrium all along this line; At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. These curves represent the relationships between phase. Equilibrium between the. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.slideshare.net
Power Point Solids & Liquids Solid And Liquid Phases Can Exist In Equilibrium Between Points Several distinct lines separate these phases. The solid and liquid phases are in equilibrium all along this line; In figure \(\pageindex{1}\), the line that. The line that connects points a and b is the. These curves represent the relationships between phase. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.slideserve.com
PPT Solid Liquid Phase Diagrams PowerPoint Presentation, free Solid And Liquid Phases Can Exist In Equilibrium Between Points In figure \(\pageindex{1}\), the line that. The line that connects points a and b is the. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. The solid and liquid phases are in equilibrium all along this line; The lines in. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Liquids and Solids Chapter ppt download Solid And Liquid Phases Can Exist In Equilibrium Between Points Several distinct lines separate these phases. The line that connects points a and b is the. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. These curves represent the relationships between phase. Equilibrium between the liquid and gas phase will exist. At the phase transition, such as the boiling point between liquid and gas. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Phase Changes. ppt download Solid And Liquid Phases Can Exist In Equilibrium Between Points Equilibrium between the liquid and gas phase will exist. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. Several distinct lines separate these phases. The line that connects points a and b is the. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.scienceabc.com
Can All Elements Directly Transition From Solid To Gas? » ScienceABC Solid And Liquid Phases Can Exist In Equilibrium Between Points These curves represent the relationships between phase. The line that connects points a and b is the. Crossing the line horizontally corresponds to melting or freezing. Several distinct lines separate these phases. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From courses.lumenlearning.com
Phase Diagrams Chemistry Solid And Liquid Phases Can Exist In Equilibrium Between Points The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. These curves represent the relationships between phase. The solid and liquid phases are in equilibrium all along this line; In figure \(\pageindex{1}\), the line that. Crossing the line horizontally corresponds to melting or freezing. Equilibrium between the liquid and gas phase will exist. At the. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.youtube.com
Solidliquid phase diagrams YouTube Solid And Liquid Phases Can Exist In Equilibrium Between Points The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. Several distinct lines separate these phases. Equilibrium between the liquid and gas phase will exist. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. At the phase transition, such as the boiling. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.slideserve.com
PPT Phase Equilibrium PowerPoint Presentation, free download ID2503286 Solid And Liquid Phases Can Exist In Equilibrium Between Points These curves represent the relationships between phase. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. Crossing the line horizontally corresponds to melting or freezing. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. The solid and liquid phases are in. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Solid And Liquid Phases Can Exist In Equilibrium Between Points Crossing the line horizontally corresponds to melting or freezing. Equilibrium between the liquid and gas phase will exist. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.researchgate.net
Equilibrium concentrations of the solid and the liquid phase for the Solid And Liquid Phases Can Exist In Equilibrium Between Points The solid and liquid phases are in equilibrium all along this line; Equilibrium between the liquid and gas phase will exist. The line that connects points a and b is the. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. Crossing the line horizontally corresponds to melting. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From x-engineer.org
Which are the states of matter Solid And Liquid Phases Can Exist In Equilibrium Between Points The solid and liquid phases are in equilibrium all along this line; Crossing the line horizontally corresponds to melting or freezing. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. Equilibrium between the liquid and gas phase will exist. The line that connects points a and b. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid And Liquid Phases Can Exist In Equilibrium Between Points Several distinct lines separate these phases. These curves represent the relationships between phase. The solid and liquid phases are in equilibrium all along this line; Equilibrium between the liquid and gas phase will exist. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. In figure \(\pageindex{1}\), the. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.britannica.com
phase Definition & Facts Britannica Solid And Liquid Phases Can Exist In Equilibrium Between Points Equilibrium between the liquid and gas phase will exist. Several distinct lines separate these phases. In figure \(\pageindex{1}\), the line that. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. The solid. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From studylib.net
Solid Liquid Vapor equilibria phase diagrams Solid And Liquid Phases Can Exist In Equilibrium Between Points In figure \(\pageindex{1}\), the line that. Several distinct lines separate these phases. These curves represent the relationships between phase. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. The established pressure in the gas phase is referred to as the. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From wisc.pb.unizin.org
Features of Phase Diagrams (M11Q1) UWMadison Chemistry 103/104 Solid And Liquid Phases Can Exist In Equilibrium Between Points The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. In figure \(\pageindex{1}\), the line that. Equilibrium between the liquid and gas phase will exist. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. Several distinct lines separate these phases. Crossing the. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Phase Diagrams, Structures of Solids, Bonding in Solids ppt download Solid And Liquid Phases Can Exist In Equilibrium Between Points The line that connects points a and b is the. Several distinct lines separate these phases. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. The solid and liquid phases are in equilibrium all along this line; These curves represent. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.snexplores.org
Explainer What are the different states of matter? Solid And Liquid Phases Can Exist In Equilibrium Between Points Several distinct lines separate these phases. Equilibrium between the liquid and gas phase will exist. The solid and liquid phases are in equilibrium all along this line; At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. These curves represent the. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From www.britannica.com
phase Definition & Facts Britannica Solid And Liquid Phases Can Exist In Equilibrium Between Points Crossing the line horizontally corresponds to melting or freezing. The solid and liquid phases are in equilibrium all along this line; Equilibrium between the liquid and gas phase will exist. The line that connects points a and b is the. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From brainly.com
Under certain conditions,the solid and liquid states of water can exist Solid And Liquid Phases Can Exist In Equilibrium Between Points In figure \(\pageindex{1}\), the line that. At the phase transition, such as the boiling point between liquid and gas phases, the two states of matter have identical free energies and are equally likely to exist. These curves represent the relationships between phase. The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. The line that. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Intermolecular Forces and Liquids and Solids ppt download Solid And Liquid Phases Can Exist In Equilibrium Between Points Several distinct lines separate these phases. The line that connects points a and b is the. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. The solid and liquid phases are in equilibrium all along this line; Crossing the line horizontally corresponds to melting or freezing. At. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Phases of Matter Particle Nature of Matter and Changes of State 4 ppt Solid And Liquid Phases Can Exist In Equilibrium Between Points The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. The line that connects points a and b is the. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. Several distinct lines separate these phases. Crossing the line horizontally corresponds to melting. Solid And Liquid Phases Can Exist In Equilibrium Between Points.
From slideplayer.com
Intermolecular Forces, Liquids, and Solids ppt download Solid And Liquid Phases Can Exist In Equilibrium Between Points Crossing the line horizontally corresponds to melting or freezing. The solid and liquid phases are in equilibrium all along this line; The established pressure in the gas phase is referred to as the equilibrium vapor pressure,. Several distinct lines separate these phases. These curves represent the relationships between phase. At the phase transition, such as the boiling point between liquid. Solid And Liquid Phases Can Exist In Equilibrium Between Points.