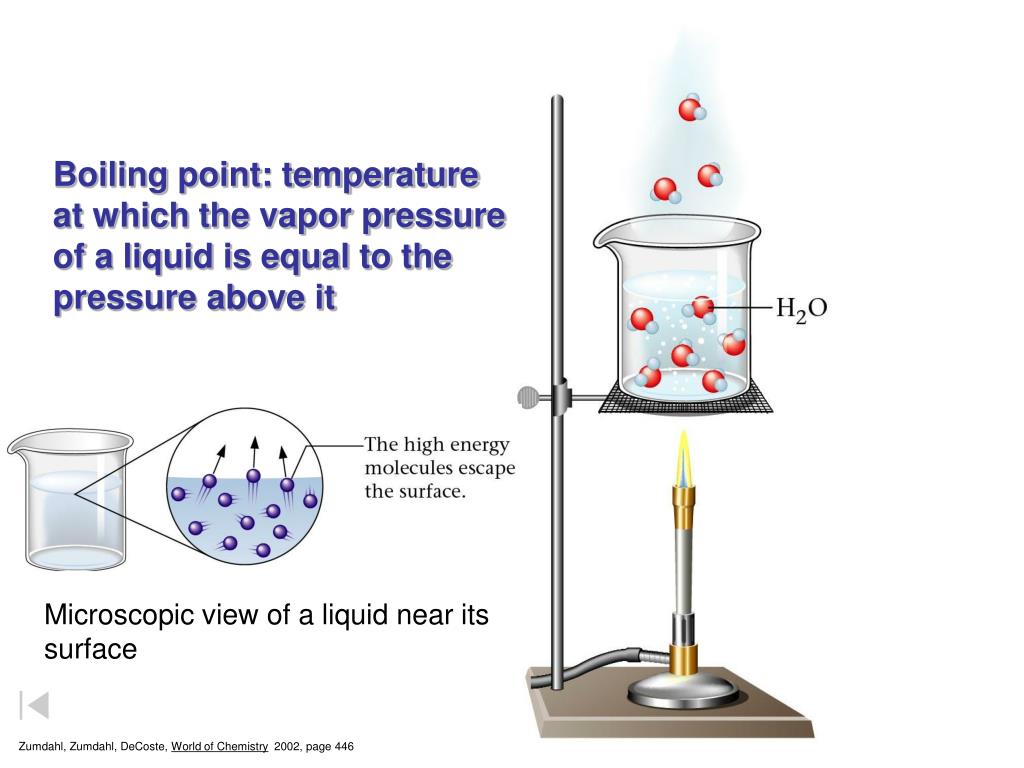
What Is The Boiling Point Of Water When Salt Is Added . When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. Why does the salted water boil more quickly, even though it has a higher boiling point? If you add salt to water, you raise the boiling point, or the temperature at which water boils. Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. Find answers to common questions. Find out how salt affects the boiling point of water and why you should add salt before. If your concentrations of salt are. Find out how this phenomenon is. Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor pressure. Learn why adding salt to water does not lower its boiling point, but increases it slightly. This phenomenon is known as boiling point. The boiling point of water increases when salt is dissolved in it. The temperature needed to boil will. It's because adding the salt lowered the heat capacity of the water.
from www.slideserve.com
Learn why adding salt to water does not lower its boiling point, but increases it slightly. It's because adding the salt lowered the heat capacity of the water. If you add salt to water, you raise the boiling point, or the temperature at which water boils. If your concentrations of salt are. Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. Find out how salt affects the boiling point of water and why you should add salt before. Find out how this phenomenon is. Find answers to common questions. The boiling point of water increases when salt is dissolved in it. The temperature needed to boil will.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
What Is The Boiling Point Of Water When Salt Is Added This phenomenon is known as boiling point. Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. This phenomenon is known as boiling point. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Why does the salted water boil more quickly, even though it has a higher boiling point? If your concentrations of salt are. Find out how salt affects the boiling point of water and why you should add salt before. Find answers to common questions. Learn why adding salt to water does not lower its boiling point, but increases it slightly. Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor pressure. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Find out how this phenomenon is. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. It's because adding the salt lowered the heat capacity of the water. The temperature needed to boil will. The boiling point of water increases when salt is dissolved in it.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is The Boiling Point Of Water When Salt Is Added When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point is raised by 0.5 degrees celsius for water with 29.2. What Is The Boiling Point Of Water When Salt Is Added.
From www.researchgate.net
Melting and boiling points of select salt compounds Download Table What Is The Boiling Point Of Water When Salt Is Added This phenomenon is known as boiling point. Find out how this phenomenon is. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Find out how salt affects the boiling point of water and why you should add salt before. Learn. What Is The Boiling Point Of Water When Salt Is Added.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube What Is The Boiling Point Of Water When Salt Is Added The temperature needed to boil will. It's because adding the salt lowered the heat capacity of the water. If your concentrations of salt are. Find out how salt affects the boiling point of water and why you should add salt before. This phenomenon is known as boiling point. The boiling point is raised by 0.5 degrees celsius for water with. What Is The Boiling Point Of Water When Salt Is Added.
From mavink.com
Water Boiling Point Pressure Chart What Is The Boiling Point Of Water When Salt Is Added The boiling point of water increases when salt is dissolved in it. Why does the salted water boil more quickly, even though it has a higher boiling point? Find out how this phenomenon is. Learn why adding salt to water does not lower its boiling point, but increases it slightly. When salt is added, it makes it harder for the. What Is The Boiling Point Of Water When Salt Is Added.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is The Boiling Point Of Water When Salt Is Added Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. The temperature needed to boil will. The boiling point of water increases when salt is dissolved in it. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. It's. What Is The Boiling Point Of Water When Salt Is Added.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is The Boiling Point Of Water When Salt Is Added The boiling point of water increases when salt is dissolved in it. Learn why adding salt to water does not lower its boiling point, but increases it slightly. Find out how this phenomenon is. Why does the salted water boil more quickly, even though it has a higher boiling point? The boiling point is raised by 0.5 degrees celsius for. What Is The Boiling Point Of Water When Salt Is Added.
From spikaph.blogspot.com
Boiling Point Of Water Boiling point of water YouTube For What Is The Boiling Point Of Water When Salt Is Added This phenomenon is known as boiling point. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor pressure. The boiling point is raised. What Is The Boiling Point Of Water When Salt Is Added.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Boiling Point Of Water When Salt Is Added The boiling point of water increases when salt is dissolved in it. Why does the salted water boil more quickly, even though it has a higher boiling point? Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor pressure. When salt is added, it makes it harder for the water molecules to. What Is The Boiling Point Of Water When Salt Is Added.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart What Is The Boiling Point Of Water When Salt Is Added It's because adding the salt lowered the heat capacity of the water. Learn why adding salt to water does not lower its boiling point, but increases it slightly. Find out how this phenomenon is. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. Why does the salted. What Is The Boiling Point Of Water When Salt Is Added.
From www.youtube.com
What AFFECT does salt have on the boiling point of water YouTube What Is The Boiling Point Of Water When Salt Is Added It's because adding the salt lowered the heat capacity of the water. Why does the salted water boil more quickly, even though it has a higher boiling point? The temperature needed to boil will. Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. Find answers to common questions. This phenomenon. What Is The Boiling Point Of Water When Salt Is Added.
From www.thoughtco.com
Does Adding Salt Lower the Boiling Point of Water? What Is The Boiling Point Of Water When Salt Is Added When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Find out how this phenomenon is. The boiling point of water increases when salt is dissolved in it. If your concentrations of salt are. This phenomenon is known as boiling point.. What Is The Boiling Point Of Water When Salt Is Added.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is The Boiling Point Of Water When Salt Is Added If your concentrations of salt are. Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. It's because adding the salt lowered the heat capacity of the water. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which. What Is The Boiling Point Of Water When Salt Is Added.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is The Boiling Point Of Water When Salt Is Added It's because adding the salt lowered the heat capacity of the water. The boiling point of water increases when salt is dissolved in it. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in. What Is The Boiling Point Of Water When Salt Is Added.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Boiling Point Of Water When Salt Is Added This phenomenon is known as boiling point. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. Why does the salted water boil more quickly, even though it has a higher boiling point? Find answers to common questions. Find out how this phenomenon is. Learn why adding salt. What Is The Boiling Point Of Water When Salt Is Added.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Is The Boiling Point Of Water When Salt Is Added It's because adding the salt lowered the heat capacity of the water. Learn why adding salt to water does not lower its boiling point, but increases it slightly. Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor pressure. Learn why adding salt to water does not make it boil faster, but. What Is The Boiling Point Of Water When Salt Is Added.
From surfguppy.com
Colligative Property Boiling Point Elevation What Is The Boiling Point Of Water When Salt Is Added Why does the salted water boil more quickly, even though it has a higher boiling point? If you add salt to water, you raise the boiling point, or the temperature at which water boils. If your concentrations of salt are. Learn why adding salt to water does not lower its boiling point, but increases it slightly. It's because adding the. What Is The Boiling Point Of Water When Salt Is Added.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free What Is The Boiling Point Of Water When Salt Is Added If you add salt to water, you raise the boiling point, or the temperature at which water boils. Why does the salted water boil more quickly, even though it has a higher boiling point? Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor pressure. Find out how this phenomenon is. The. What Is The Boiling Point Of Water When Salt Is Added.
From www.youtube.com
Chemistry Animation Salt in Boiling Water.wmv YouTube What Is The Boiling Point Of Water When Salt Is Added This phenomenon is known as boiling point. The boiling point of water increases when salt is dissolved in it. Why does the salted water boil more quickly, even though it has a higher boiling point? Find out how salt affects the boiling point of water and why you should add salt before. The boiling point is raised by 0.5 degrees. What Is The Boiling Point Of Water When Salt Is Added.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Boiling Point Of Water When Salt Is Added If you add salt to water, you raise the boiling point, or the temperature at which water boils. Find answers to common questions. Learn why adding salt to water does not lower its boiling point, but increases it slightly. Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. The boiling. What Is The Boiling Point Of Water When Salt Is Added.
From chart-studio.plotly.com
How Does Salt Affect the Boiling Point of Water? bar chart made by What Is The Boiling Point Of Water When Salt Is Added Find out how salt affects the boiling point of water and why you should add salt before. If your concentrations of salt are. Why does the salted water boil more quickly, even though it has a higher boiling point? It's because adding the salt lowered the heat capacity of the water. Find out how this phenomenon is. Learn why adding. What Is The Boiling Point Of Water When Salt Is Added.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is The Boiling Point Of Water When Salt Is Added The boiling point of water increases when salt is dissolved in it. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. Why does the salted water boil more quickly, even though it has a higher boiling point? When salt is added, it makes it harder for the. What Is The Boiling Point Of Water When Salt Is Added.
From www.thoughtco.com
Why Does Adding Salt Increase the Boiling Point of Water? What Is The Boiling Point Of Water When Salt Is Added If you add salt to water, you raise the boiling point, or the temperature at which water boils. If your concentrations of salt are. This phenomenon is known as boiling point. Find answers to common questions. Find out how this phenomenon is. Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor. What Is The Boiling Point Of Water When Salt Is Added.
From www.youtube.com
Lab 18.2 Adding Salt to Boiling Water YouTube What Is The Boiling Point Of Water When Salt Is Added If you add salt to water, you raise the boiling point, or the temperature at which water boils. Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. The temperature needed to boil will. Learn why adding salt to water does not lower its boiling point, but increases it slightly. This. What Is The Boiling Point Of Water When Salt Is Added.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is The Boiling Point Of Water When Salt Is Added It's because adding the salt lowered the heat capacity of the water. This phenomenon is known as boiling point. Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase,. What Is The Boiling Point Of Water When Salt Is Added.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Is The Boiling Point Of Water When Salt Is Added It's because adding the salt lowered the heat capacity of the water. Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor pressure. Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. The temperature needed to boil will. Find answers to common. What Is The Boiling Point Of Water When Salt Is Added.
From www.thoughtco.com
Why Adding Salt to Water Increases the Boiling Point What Is The Boiling Point Of Water When Salt Is Added The temperature needed to boil will. If your concentrations of salt are. Learn why adding salt to water does not lower its boiling point, but increases it slightly. Find out how salt affects the boiling point of water and why you should add salt before. When salt is added, it makes it harder for the water molecules to escape from. What Is The Boiling Point Of Water When Salt Is Added.
From hxeqcdaps.blob.core.windows.net
What Happens To The Boiling Point And Freezing Point Of Water When Salt What Is The Boiling Point Of Water When Salt Is Added Learn why adding salt to water does not make it boil faster, but actually increases the boiling point slightly. If your concentrations of salt are. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. The temperature needed to boil will.. What Is The Boiling Point Of Water When Salt Is Added.
From ar.inspiredpencil.com
Boiling Point Of Water Graph What Is The Boiling Point Of Water When Salt Is Added This phenomenon is known as boiling point. Find answers to common questions. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. It's because adding the salt lowered the heat capacity of the water. Learn why adding salt to water does. What Is The Boiling Point Of Water When Salt Is Added.
From www.perkinselearning.org
Can the Boiling Point of Water be Changed? Perkins eLearning What Is The Boiling Point Of Water When Salt Is Added Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor pressure. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will. It's because adding the salt lowered the heat capacity of the water. Find answers to common questions.. What Is The Boiling Point Of Water When Salt Is Added.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Is The Boiling Point Of Water When Salt Is Added The boiling point of water increases when salt is dissolved in it. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. It's because adding the salt lowered the heat capacity of the water. The boiling point is raised by 0.5. What Is The Boiling Point Of Water When Salt Is Added.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is The Boiling Point Of Water When Salt Is Added Learn why adding salt to water raises its boiling point and how this relates to entropy and vapor pressure. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. It's because adding the salt lowered the heat capacity of the water.. What Is The Boiling Point Of Water When Salt Is Added.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is The Boiling Point Of Water When Salt Is Added If your concentrations of salt are. Find out how this phenomenon is. Find answers to common questions. Why does the salted water boil more quickly, even though it has a higher boiling point? The temperature needed to boil will. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of. What Is The Boiling Point Of Water When Salt Is Added.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is The Boiling Point Of Water When Salt Is Added If your concentrations of salt are. The temperature needed to boil will. When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. It's because adding the salt lowered the heat capacity of the water. Find answers to common questions. Learn why. What Is The Boiling Point Of Water When Salt Is Added.
From howchimp.com
What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit What Is The Boiling Point Of Water When Salt Is Added This phenomenon is known as boiling point. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point of water increases when salt is dissolved in it. If your concentrations of salt are. Learn why adding salt to water raises its boiling point and how this relates to entropy and. What Is The Boiling Point Of Water When Salt Is Added.
From talia-well-banks.blogspot.com
Why Does Salt Increase the Boiling Point of Water What Is The Boiling Point Of Water When Salt Is Added When salt is added, it makes it harder for the water molecules to escape from the pot and enter the gas phase, which happens when water boils, giddings said. Find out how this phenomenon is. Find answers to common questions. Learn why adding salt to water does not lower its boiling point, but increases it slightly. It's because adding the. What Is The Boiling Point Of Water When Salt Is Added.