What Is A Zero Dipole Moment . dipole moment is equal to the product of the partial charge and the distance. Dipole moment of h 2 o (water) in a water molecule, the electrons. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. their vector sum is zero, so co2 therefore has no net dipole. The vector addition of the dipoles equals. The two identical atoms in each of these. The equation for dipole moment is as follows. In a molecule of co 2, the two individual. the dipole is often modelled by assigning partial charges to the atoms. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. therefore, the net dipole moment of a bef 2 molecule is zero.
from www.chegg.com
their vector sum is zero, so co2 therefore has no net dipole. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. Dipole moment of h 2 o (water) in a water molecule, the electrons. The two identical atoms in each of these. The equation for dipole moment is as follows. therefore, the net dipole moment of a bef 2 molecule is zero. The vector addition of the dipoles equals. dipole moment is equal to the product of the partial charge and the distance. In a molecule of co 2, the two individual. the dipole is often modelled by assigning partial charges to the atoms.
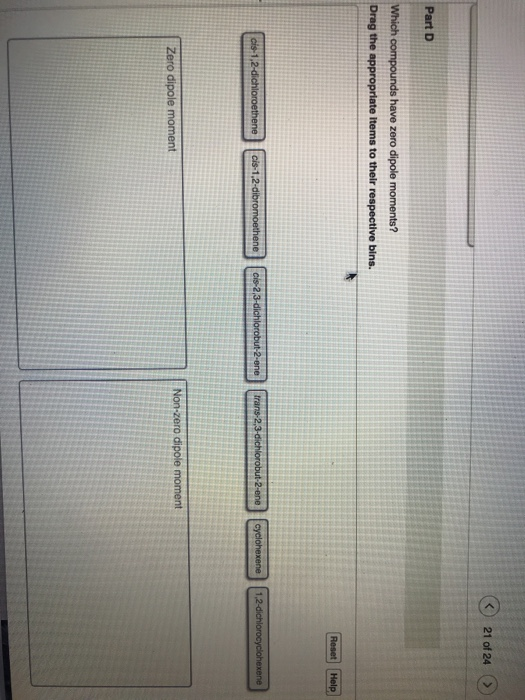
Solved c) 21 of 24 Part D Drag the appropriate Items to
What Is A Zero Dipole Moment the dipole is often modelled by assigning partial charges to the atoms. dipole moment is equal to the product of the partial charge and the distance. The equation for dipole moment is as follows. therefore, the net dipole moment of a bef 2 molecule is zero. In a molecule of co 2, the two individual. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. the dipole is often modelled by assigning partial charges to the atoms. The vector addition of the dipoles equals. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. The two identical atoms in each of these. Dipole moment of h 2 o (water) in a water molecule, the electrons. their vector sum is zero, so co2 therefore has no net dipole.
From www.youtube.com
Electric Dipole & Dipole Moment Class 12th Physics Handwritten What Is A Zero Dipole Moment Dipole moment of h 2 o (water) in a water molecule, the electrons. The two identical atoms in each of these. The equation for dipole moment is as follows. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. dipole moment is equal to the product of the partial charge and. What Is A Zero Dipole Moment.
From socratic.org
Question 5981b Socratic What Is A Zero Dipole Moment The two identical atoms in each of these. The equation for dipole moment is as follows. dipole moment is equal to the product of the partial charge and the distance. Dipole moment of h 2 o (water) in a water molecule, the electrons. the dipole is often modelled by assigning partial charges to the atoms. however, since. What Is A Zero Dipole Moment.
From www.youtube.com
Dipole moment YouTube What Is A Zero Dipole Moment therefore, the net dipole moment of a bef 2 molecule is zero. In a molecule of co 2, the two individual. the dipole is often modelled by assigning partial charges to the atoms. their vector sum is zero, so co2 therefore has no net dipole. dipole moment is equal to the product of the partial charge. What Is A Zero Dipole Moment.
From brainly.in
why the dipole moment of BF3 is 0? Brainly.in What Is A Zero Dipole Moment however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. their vector sum is zero, so co2 therefore has no net dipole. the dipole is often modelled by assigning partial charges to the atoms. therefore, the net dipole moment of a bef 2 molecule is zero. In a molecule of co. What Is A Zero Dipole Moment.
From www.youtube.com
Dipole moment of so2, h2o, ccl4, chcl3, cis & trans alkenes, co2, nh3 What Is A Zero Dipole Moment Dipole moment of h 2 o (water) in a water molecule, the electrons. The equation for dipole moment is as follows. the dipole is often modelled by assigning partial charges to the atoms. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. however, since the molecule is linear, these. What Is A Zero Dipole Moment.
From discover.hubpages.com
Lucid Understanding Of, “Dipole Moment Of A Molecule” HubPages What Is A Zero Dipole Moment dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. the dipole is often modelled by assigning partial charges to the atoms. The two identical atoms in each of these. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. In a molecule of co. What Is A Zero Dipole Moment.
From www.chemistrysteps.com
Molecular Dipole The Overall Polarity of the Molecule Chemistry Steps What Is A Zero Dipole Moment dipole moment is equal to the product of the partial charge and the distance. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. their vector sum is zero, so co2 therefore has no net dipole. The vector addition of the dipoles equals. The equation for dipole moment is as. What Is A Zero Dipole Moment.
From www.chegg.com
Solved c) 21 of 24 Part D Drag the appropriate Items to What Is A Zero Dipole Moment however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. Dipole moment of h 2 o (water) in a water molecule, the electrons. dipole moment is equal to the product of the partial charge and the distance. The equation for dipole moment is as follows. The two identical atoms in each of these.. What Is A Zero Dipole Moment.
From www.youtube.com
Super Easy Trick for Dipole Moment How to Find Dipole Moment in 2 What Is A Zero Dipole Moment The two identical atoms in each of these. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. therefore, the net dipole moment of a bef 2 molecule is zero. Dipole moment of h 2 o (water) in a water molecule, the electrons. however, since the molecule is linear, these. What Is A Zero Dipole Moment.
From brainly.in
The which molecule has zero dipole moment Brainly.in What Is A Zero Dipole Moment their vector sum is zero, so co2 therefore has no net dipole. The vector addition of the dipoles equals. the dipole is often modelled by assigning partial charges to the atoms. therefore, the net dipole moment of a bef 2 molecule is zero. In a molecule of co 2, the two individual. dipole moment is equal. What Is A Zero Dipole Moment.
From www.youtube.com
Dipole Dipole Forces of Attraction Intermolecular Forces YouTube What Is A Zero Dipole Moment dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. The equation for dipole moment is as follows. their vector sum is zero, so co2 therefore has no net dipole. the dipole is often modelled by assigning partial charges to the atoms. dipole moment is equal to the product. What Is A Zero Dipole Moment.
From chem.libretexts.org
Dipole Moments Chemistry LibreTexts What Is A Zero Dipole Moment The equation for dipole moment is as follows. dipole moment is equal to the product of the partial charge and the distance. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. The vector addition of the dipoles equals. The two identical atoms in each of these. their vector sum. What Is A Zero Dipole Moment.
From slideplayer.com
Molecular Polarity Molecular Structure ppt video online download What Is A Zero Dipole Moment The vector addition of the dipoles equals. therefore, the net dipole moment of a bef 2 molecule is zero. The equation for dipole moment is as follows. the dipole is often modelled by assigning partial charges to the atoms. In a molecule of co 2, the two individual. dipole moment is equal to the product of the. What Is A Zero Dipole Moment.
From www.chemistrysteps.com
Molecular Dipole The Overall Polarity of the Molecule Chemistry Steps What Is A Zero Dipole Moment therefore, the net dipole moment of a bef 2 molecule is zero. In a molecule of co 2, the two individual. The vector addition of the dipoles equals. the dipole is often modelled by assigning partial charges to the atoms. dipole moment is equal to the product of the partial charge and the distance. The two identical. What Is A Zero Dipole Moment.
From www.slideserve.com
PPT Lecture 21 Ionic to Covalent PowerPoint Presentation, free What Is A Zero Dipole Moment The equation for dipole moment is as follows. In a molecule of co 2, the two individual. The vector addition of the dipoles equals. dipole moment is equal to the product of the partial charge and the distance. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. their vector. What Is A Zero Dipole Moment.
From byjus.com
23.Dipole moment of BeF2 and BF3? What Is A Zero Dipole Moment therefore, the net dipole moment of a bef 2 molecule is zero. In a molecule of co 2, the two individual. Dipole moment of h 2 o (water) in a water molecule, the electrons. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. The two identical atoms in each of these. . What Is A Zero Dipole Moment.
From brainly.in
Explain dipole dipole forces Brainly.in What Is A Zero Dipole Moment therefore, the net dipole moment of a bef 2 molecule is zero. their vector sum is zero, so co2 therefore has no net dipole. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. the dipole is often modelled by assigning partial charges to the atoms. Dipole moment of. What Is A Zero Dipole Moment.
From www.expii.com
Dipole Moment — Definition & Overview Expii What Is A Zero Dipole Moment The vector addition of the dipoles equals. The equation for dipole moment is as follows. Dipole moment of h 2 o (water) in a water molecule, the electrons. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. their vector sum is zero, so co2 therefore has no net dipole. therefore, the. What Is A Zero Dipole Moment.
From www.sccsikar.com
Electric dipole and dipole Moment SCIENCE CAREER COACHING What Is A Zero Dipole Moment their vector sum is zero, so co2 therefore has no net dipole. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. therefore, the net dipole moment of a bef 2 molecule is zero. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e.. What Is A Zero Dipole Moment.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID6836286 What Is A Zero Dipole Moment Dipole moment of h 2 o (water) in a water molecule, the electrons. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. therefore, the net dipole moment of a bef 2 molecule is zero. dipole moment is equal to the product of the partial charge and the distance. . What Is A Zero Dipole Moment.
From notariaurbina.cl
cerinţe Praf siluetă dipole moment calculation Orb Înşelăciune violă What Is A Zero Dipole Moment their vector sum is zero, so co2 therefore has no net dipole. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. The vector addition of the dipoles equals. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. Dipole moment of h 2 o. What Is A Zero Dipole Moment.
From byjus.com
9. What is dipole? What is zero and non zero dipole moment? What Is A Zero Dipole Moment dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. dipole moment is equal to the product of the partial charge and the distance. In a molecule of co 2, the two individual. their vector sum is zero, so co2 therefore has no net dipole. The two identical atoms in. What Is A Zero Dipole Moment.
From alexusfersmeza.blogspot.com
Does Sif4 Have a Dipole Moment What Is A Zero Dipole Moment therefore, the net dipole moment of a bef 2 molecule is zero. the dipole is often modelled by assigning partial charges to the atoms. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e.. What Is A Zero Dipole Moment.
From elainexiwalters.blogspot.com
What Causes a Molecule to Have a Net Dipole Moment What Is A Zero Dipole Moment The vector addition of the dipoles equals. therefore, the net dipole moment of a bef 2 molecule is zero. Dipole moment of h 2 o (water) in a water molecule, the electrons. The two identical atoms in each of these. In a molecule of co 2, the two individual. the dipole is often modelled by assigning partial charges. What Is A Zero Dipole Moment.
From brainly.in
which of the following has zero dipolemoment? a) ClF b) PCl3 c) SiF4 d What Is A Zero Dipole Moment The equation for dipole moment is as follows. The two identical atoms in each of these. their vector sum is zero, so co2 therefore has no net dipole. The vector addition of the dipoles equals. therefore, the net dipole moment of a bef 2 molecule is zero. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride. What Is A Zero Dipole Moment.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Is A Zero Dipole Moment however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. dipole moment is equal to the product of the partial charge and the distance. The two identical atoms in each of these. their vector sum is zero, so co2 therefore has no net dipole. therefore, the net dipole moment of a. What Is A Zero Dipole Moment.
From www.toppr.com
Which molecule has zero dipole moment? What Is A Zero Dipole Moment dipole moment is equal to the product of the partial charge and the distance. therefore, the net dipole moment of a bef 2 molecule is zero. The vector addition of the dipoles equals. The equation for dipole moment is as follows. Dipole moment of h 2 o (water) in a water molecule, the electrons. The two identical atoms. What Is A Zero Dipole Moment.
From byjus.com
28. What is the dipole moment order for (CH3Cl);(CH2Cl2);(CHCl3);(CCl4) What Is A Zero Dipole Moment therefore, the net dipole moment of a bef 2 molecule is zero. their vector sum is zero, so co2 therefore has no net dipole. the dipole is often modelled by assigning partial charges to the atoms. Dipole moment of h 2 o (water) in a water molecule, the electrons. The vector addition of the dipoles equals. The. What Is A Zero Dipole Moment.
From hacfoot.weebly.com
Ion bonding hydrogen bonding dipole dipole hacfoot What Is A Zero Dipole Moment dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. The vector addition of the dipoles equals. therefore, the net dipole moment of a bef 2 molecule is zero. In a molecule of co 2, the two individual. Dipole moment of h 2 o (water) in a water molecule, the electrons.. What Is A Zero Dipole Moment.
From chemizi.blogspot.com
dipole moment bond moment group moment and Influence of dipole moment What Is A Zero Dipole Moment therefore, the net dipole moment of a bef 2 molecule is zero. their vector sum is zero, so co2 therefore has no net dipole. In a molecule of co 2, the two individual. The vector addition of the dipoles equals. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. the. What Is A Zero Dipole Moment.
From brainly.in
Explain the beh2has zero dipole moment although beh bond are polar What Is A Zero Dipole Moment The two identical atoms in each of these. dipole moment in \(bh_3\) and \(nh_3\) in the boron trihydride \(bh_3\) molecule, the dipole moment is zero. dipole moment is equal to the product of the partial charge and the distance. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. In a molecule. What Is A Zero Dipole Moment.
From www.youtube.com
Define dipole moment of an electric dipole. Is it a scalar quantity or What Is A Zero Dipole Moment The equation for dipole moment is as follows. The vector addition of the dipoles equals. the dipole is often modelled by assigning partial charges to the atoms. therefore, the net dipole moment of a bef 2 molecule is zero. dipole moment is equal to the product of the partial charge and the distance. dipole moment in. What Is A Zero Dipole Moment.
From classnotes.org.in
Dipole Moments Chemical Bonding and Molecular Structure, Chemistry What Is A Zero Dipole Moment Dipole moment of h 2 o (water) in a water molecule, the electrons. The vector addition of the dipoles equals. The two identical atoms in each of these. therefore, the net dipole moment of a bef 2 molecule is zero. their vector sum is zero, so co2 therefore has no net dipole. In a molecule of co 2,. What Is A Zero Dipole Moment.
From www.youtube.com
Dipole moment of which compound will zero ? YouTube What Is A Zero Dipole Moment In a molecule of co 2, the two individual. however, since the molecule is linear, these two bond dipoles cancel each other out (i.e. Dipole moment of h 2 o (water) in a water molecule, the electrons. The equation for dipole moment is as follows. The vector addition of the dipoles equals. dipole moment is equal to the. What Is A Zero Dipole Moment.
From www.flexiprep.com
Chemistry Class 11 NCERT Solutions Chapter 4 Chemical Bonding and What Is A Zero Dipole Moment dipole moment is equal to the product of the partial charge and the distance. The vector addition of the dipoles equals. therefore, the net dipole moment of a bef 2 molecule is zero. In a molecule of co 2, the two individual. The two identical atoms in each of these. The equation for dipole moment is as follows.. What Is A Zero Dipole Moment.