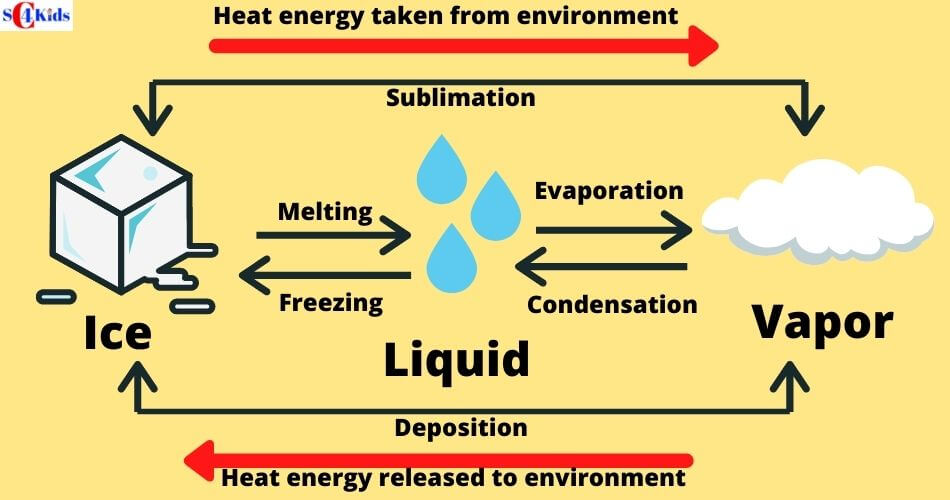
Solid To Liquid Latent Heat . Melting of ice occurs in two steps: Equivalently, the energy liberated when one unit of a substance transitions from liquid to solid. Heat is transferred from the soda to the ice for melting. The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; Melting of ice occurs in two. The energy required to transition one unit of a substance from solid to liquid; A change from a liquid to a gaseous phase is an example of a phase transition. That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Another common phase transition is from a solid to a liquid phase. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The ice cubes are at the melting temperature of \ (0^oc\). Heat is transferred from the soda to the ice for melting. First the phase change occurs and solid (ice).
from smartclass4kids.com
A change from a liquid to a gaseous phase is an example of a phase transition. First the phase change occurs and solid (ice). That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Heat is transferred from the soda to the ice for melting. The energy required to transition one unit of a substance from solid to liquid; The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Melting of ice occurs in two steps: Melting of ice occurs in two. Another common phase transition is from a solid to a liquid phase.
Changing States of Matter Solid, Liquid,Gas, Phase Change
Solid To Liquid Latent Heat Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Melting of ice occurs in two. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. Heat is transferred from the soda to the ice for melting. Another common phase transition is from a solid to a liquid phase. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The energy required to transition one unit of a substance from solid to liquid; Equivalently, the energy liberated when one unit of a substance transitions from liquid to solid. Specific latent heat is the amount of. The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. Melting of ice occurs in two steps: The latent heat of vaporization is the. A change from a liquid to a gaseous phase is an example of a phase transition. Heat is transferred from the soda to the ice for melting. The ice cubes are at the melting temperature of \ (0^oc\).
From alevelphysicsnotes.com
Mr Toogood Physics Latent heat Solid To Liquid Latent Heat The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Heat is transferred from the soda to the ice for melting. First the phase change occurs and solid (ice).. Solid To Liquid Latent Heat.
From dokumen.tips
(PPT) 4.3 SPECIFIC LATENT HEAT. HEAT SPECIFIC HEAT CAPACITY LATENT HEAT Solid To Liquid Latent Heat Melting of ice occurs in two steps: Heat is transferred from the soda to the ice for melting. Melting of ice occurs in two. Specific latent heat is the amount of. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Equivalently, the energy liberated when one unit of a. Solid To Liquid Latent Heat.
From www.slideserve.com
PPT Phase of Water and Latent Heats PowerPoint Presentation, free Solid To Liquid Latent Heat The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Heat is transferred from the soda to the ice for melting. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Energy is required to melt a. Solid To Liquid Latent Heat.
From www.researchgate.net
Latent Heat of Storage from Solid to Liquid. Download Scientific Diagram Solid To Liquid Latent Heat Specific latent heat is the amount of. Equivalently, the energy liberated when one unit of a substance transitions from liquid to solid. A change from a liquid to a gaseous phase is an example of a phase transition. Melting of ice occurs in two steps: Energy is required to melt a solid because the cohesive bonds between the molecules in. Solid To Liquid Latent Heat.
From slideplayer.com
1st Law of Thermodynamics Heat Transfer ppt download Solid To Liquid Latent Heat Another common phase transition is from a solid to a liquid phase. Heat is transferred from the soda to the ice for melting. First the phase change occurs and solid (ice). Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. A change from a. Solid To Liquid Latent Heat.
From slideplayer.com
Heat, heat transport and weather ppt download Solid To Liquid Latent Heat First the phase change occurs and solid (ice). The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Heat is transferred from the soda to the ice for melting. Melting of ice occurs in two steps: Latent heat, energy absorbed or released by a substance during a change in its. Solid To Liquid Latent Heat.
From nisa-wr.blogspot.com
Latent Heat Of Fusion Changes of state / The latent heat of fusion is Solid To Liquid Latent Heat The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; Equivalently, the energy liberated when one unit of a substance transitions from liquid to solid. First the phase change occurs and solid (ice). Heat is transferred from the soda to the ice for melting. Latent heat, energy absorbed or released by a. Solid To Liquid Latent Heat.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Solid To Liquid Latent Heat Another common phase transition is from a solid to a liquid phase. Equivalently, the energy liberated when one unit of a substance transitions from liquid to solid. A change from a liquid to a gaseous phase is an example of a phase transition. The energy required to transition one unit of a substance from solid to liquid; Heat is transferred. Solid To Liquid Latent Heat.
From www.ces.fau.edu
Climate Science Investigations South Florida Energy The Driver of Solid To Liquid Latent Heat The ice cubes are at the melting temperature of \ (0^oc\). Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; Another common phase transition is from. Solid To Liquid Latent Heat.
From www.pinterest.com
Latent Heat the heat energy that must be absorbed when a substance Solid To Liquid Latent Heat Specific latent heat is the amount of. Another common phase transition is from a solid to a liquid phase. The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; First the phase change occurs and solid (ice). The latent heat of vaporization is the. Energy is required to melt a solid because. Solid To Liquid Latent Heat.
From www.fizzics.org
Specific Latent Heat notes and video lesson The Fizzics Organization Solid To Liquid Latent Heat Another common phase transition is from a solid to a liquid phase. Melting of ice occurs in two. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its. Solid To Liquid Latent Heat.
From www.intechopen.com
Figure 1. Solid To Liquid Latent Heat A change from a liquid to a gaseous phase is an example of a phase transition. The ice cubes are at the melting temperature of \ (0^oc\). Melting of ice occurs in two. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. Specific latent. Solid To Liquid Latent Heat.
From www.researchgate.net
Theoretical latent heat curve for solid/liquid phase transition of a Solid To Liquid Latent Heat That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. Another common phase transition is from a solid to a liquid phase. Heat is transferred from the soda to the ice for melting. Specific latent heat is the amount of. Melting of ice occurs in two steps: Latent heat, energy absorbed or. Solid To Liquid Latent Heat.
From www.savemyexams.com
Specific Latent Heat AQA GCSE Physics Revision Notes 2018 Solid To Liquid Latent Heat That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. The energy required to transition one unit of a substance from solid to liquid; The ice cubes are at the melting temperature of \ (0^oc\). Heat is transferred from the soda to the ice for melting. Energy is required to melt a. Solid To Liquid Latent Heat.
From slideplayer.com
Heat, Temperature, Heat Transfer, Thermal Expansion & Thermodynamics Solid To Liquid Latent Heat Specific latent heat is the amount of. Melting of ice occurs in two steps: Heat is transferred from the soda to the ice for melting. The ice cubes are at the melting temperature of \ (0^oc\). Melting of ice occurs in two. Another common phase transition is from a solid to a liquid phase. Latent heat, energy absorbed or released. Solid To Liquid Latent Heat.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Solid To Liquid Latent Heat Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Equivalently, the energy liberated when one unit of a substance transitions from liquid to solid. The ice cubes are at the melting temperature of \ (0^oc\). Specific latent heat is the amount of. The latent heat of. Solid To Liquid Latent Heat.
From www.researchgate.net
Classification of latent heat materials with solidliquid phase change Solid To Liquid Latent Heat Another common phase transition is from a solid to a liquid phase. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. Melting of ice occurs in two. The latent heat associated with melting a solid or freezing a liquid is called the heat of. Solid To Liquid Latent Heat.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID5769198 Solid To Liquid Latent Heat A change from a liquid to a gaseous phase is an example of a phase transition. The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. Energy is required to melt a. Solid To Liquid Latent Heat.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid To Liquid Latent Heat Another common phase transition is from a solid to a liquid phase. Melting of ice occurs in two steps: Heat is transferred from the soda to the ice for melting. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The ice cubes are at. Solid To Liquid Latent Heat.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID8874124 Solid To Liquid Latent Heat First the phase change occurs and solid (ice). Melting of ice occurs in two. The latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; The ice cubes are at the melting temperature. Solid To Liquid Latent Heat.
From slideplayer.com
Chapter 19 Heat and the First Law of Thermodynamics ppt download Solid To Liquid Latent Heat Melting of ice occurs in two steps: Heat is transferred from the soda to the ice for melting. Another common phase transition is from a solid to a liquid phase. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. The latent heat associated with melting a. Solid To Liquid Latent Heat.
From www.slideshare.net
Unit 1 Phase Changes Solid To Liquid Latent Heat Heat is transferred from the soda to the ice for melting. Melting of ice occurs in two. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The latent heat of fusion is the amount of heat needed to cause a phase change between solid. Solid To Liquid Latent Heat.
From www.slideserve.com
PPT Latent Heat PowerPoint Presentation, free download ID310809 Solid To Liquid Latent Heat The energy required to transition one unit of a substance from solid to liquid; A change from a liquid to a gaseous phase is an example of a phase transition. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Heat is transferred from the soda to. Solid To Liquid Latent Heat.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID5759037 Solid To Liquid Latent Heat The energy required to transition one unit of a substance from solid to liquid; First the phase change occurs and solid (ice). Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. Equivalently, the energy liberated when one unit of a substance transitions from liquid. Solid To Liquid Latent Heat.
From www.slideserve.com
PPT Phase of Water and Latent Heats PowerPoint Presentation, free Solid To Liquid Latent Heat The energy required to transition one unit of a substance from solid to liquid; The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The latent heat. Solid To Liquid Latent Heat.
From www.researchgate.net
Latent Heat of Storage from Solid to Liquid. Download Scientific Diagram Solid To Liquid Latent Heat That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. A change from a liquid to a gaseous phase is an example of a phase transition. Heat. Solid To Liquid Latent Heat.
From www.youtube.com
SOLIDS LIQUIDS GASES & LATENT HEAT 9th CLASS STATE/CBSE YouTube Solid To Liquid Latent Heat The energy required to transition one unit of a substance from solid to liquid; Heat is transferred from the soda to the ice for melting. Another common phase transition is from a solid to a liquid phase. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that,. Solid To Liquid Latent Heat.
From sciencepickle.com
Latent Heat Science Pickle Solid To Liquid Latent Heat Another common phase transition is from a solid to a liquid phase. A change from a liquid to a gaseous phase is an example of a phase transition. First the phase change occurs and solid (ice). That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. The latent heat of fusion is. Solid To Liquid Latent Heat.
From slideplayer.com
Lesson 7 Just a Phase. ppt download Solid To Liquid Latent Heat The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; A change from a liquid to a gaseous phase is an example of a phase transition. Melting of ice occurs in two steps: Another common phase transition is from a solid to a liquid phase. Energy is required to melt a solid. Solid To Liquid Latent Heat.
From www.teachoo.com
Latent Heat of Vaporization and Fusion Definition Teachoo Solid To Liquid Latent Heat That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; Melting of ice occurs in two. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that. Solid To Liquid Latent Heat.
From pressbooks.bccampus.ca
5.5 Phase Change and Latent Heat Douglas College Physics 1207 Solid To Liquid Latent Heat Another common phase transition is from a solid to a liquid phase. The energy required to transition one unit of a substance from solid to liquid; Melting of ice occurs in two. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. The ice cubes. Solid To Liquid Latent Heat.
From slideplayer.com
Chapter 3 States of Matter ppt download Solid To Liquid Latent Heat Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the. Another common phase transition is from a solid to a liquid phase. Heat is transferred from the soda to the ice for melting. The latent heat of fusion is the amount of heat needed to. Solid To Liquid Latent Heat.
From guides.co
States of Matter FD202 Fundamentals of Fire and Combustion on Guides Solid To Liquid Latent Heat Melting of ice occurs in two steps: The ice cubes are at the melting temperature of \ (0^oc\). Melting of ice occurs in two. A change from a liquid to a gaseous phase is an example of a phase transition. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without. Solid To Liquid Latent Heat.
From www.slideserve.com
PPT The Nature of Water PowerPoint Presentation, free download ID Solid To Liquid Latent Heat That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. First the phase change occurs and solid (ice). Melting of ice occurs in two steps: Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Another common phase transition. Solid To Liquid Latent Heat.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Solid To Liquid Latent Heat Another common phase transition is from a solid to a liquid phase. Heat is transferred from the soda to the ice for melting. First the phase change occurs and solid (ice). The latent heat of vaporization is the. That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of. A change from a. Solid To Liquid Latent Heat.