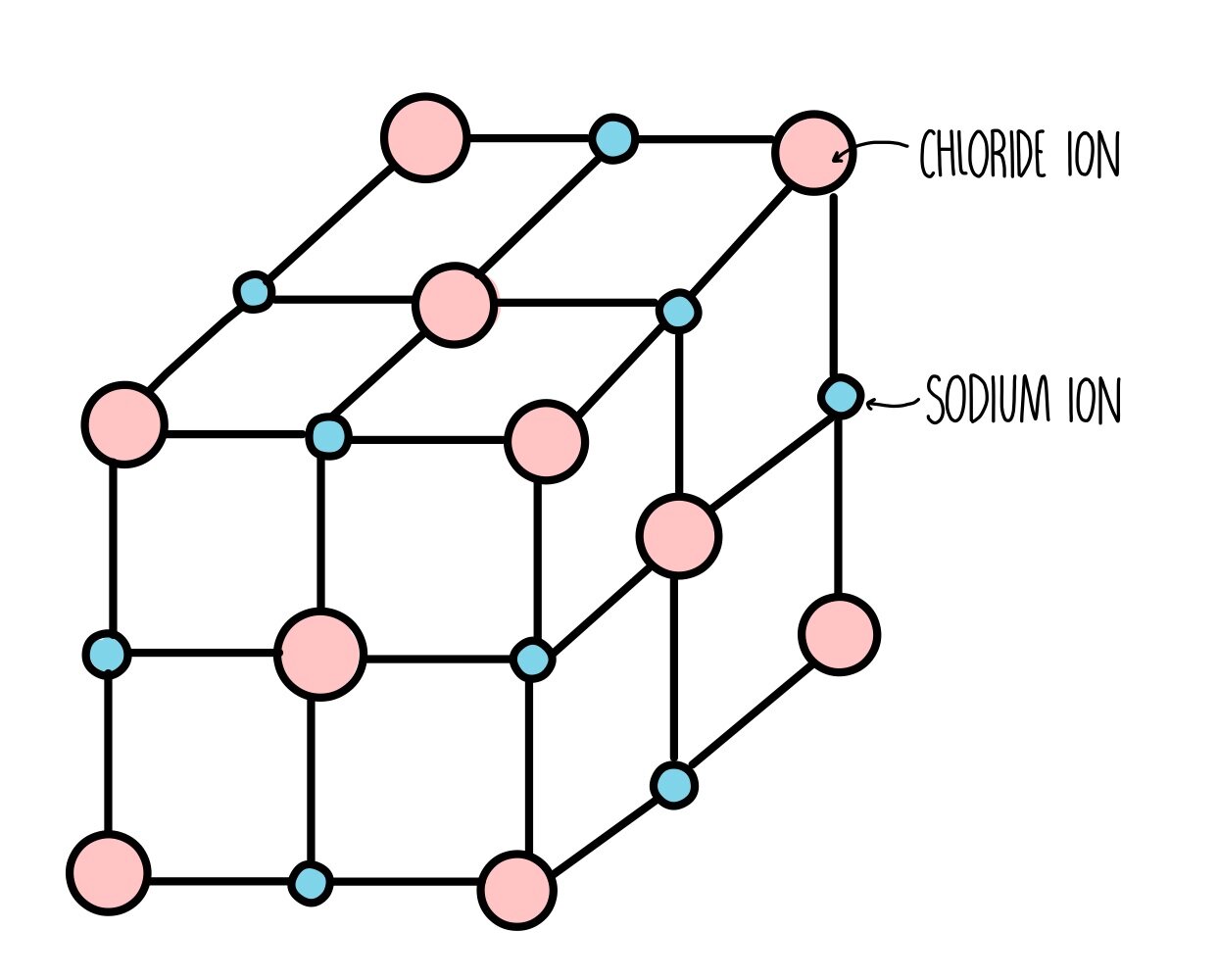
Ionic Crystal Examples . there are four types of crystals: Make a rough sketch that describes the structure of solid sodium chloride. Explain what features of a crystal are reflected in. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Properties and several examples of each type. examples of ionic crystal structures. ionic crystals are a unique structure created when two ions electrically attract to one another. list the properties of ionic crystals, and relate them to the lattice energy. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. Caesium chloride (cscl) is an ionic compound with a simple cubic lattice.
from www.thesciencehive.co.uk
Make a rough sketch that describes the structure of solid sodium chloride. Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Explain what features of a crystal are reflected in. list the properties of ionic crystals, and relate them to the lattice energy. Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. there are four types of crystals: what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? examples of ionic crystal structures. ionic crystals are a unique structure created when two ions electrically attract to one another.
Ionic Bonding — the science hive
Ionic Crystal Examples Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Explain what features of a crystal are reflected in. there are four types of crystals: list the properties of ionic crystals, and relate them to the lattice energy. ionic crystals are a unique structure created when two ions electrically attract to one another. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. Make a rough sketch that describes the structure of solid sodium chloride. examples of ionic crystal structures. Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Properties and several examples of each type.
From www.slideserve.com
PPT Ionic Bonding Part I PowerPoint Presentation, free download ID Ionic Crystal Examples examples of ionic crystal structures. Properties and several examples of each type. Make a rough sketch that describes the structure of solid sodium chloride. ionic crystals are a unique structure created when two ions electrically attract to one another. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. list the properties of ionic crystals, and relate. Ionic Crystal Examples.
From www.slideserve.com
PPT Bonding Considerations PowerPoint Presentation, free download Ionic Crystal Examples Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. ionic crystals are a unique structure created when two ions electrically attract to one another. list the properties of ionic crystals, and relate them to the lattice energy. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. what. Ionic Crystal Examples.
From chemistnotes.com
Ionic Crystal, Examples, and Properties Chemistry Notes Ionic Crystal Examples examples of ionic crystal structures. Make a rough sketch that describes the structure of solid sodium chloride. Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Properties and several examples of each type. ionic crystals are a unique structure created when two ions electrically attract to one another. Explain. Ionic Crystal Examples.
From www.shutterstock.com
Vektor Stok Crystal Lattice Structure Ionic Compounds Ionic (Tanpa Ionic Crystal Examples (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Properties and several examples of each type. ionic crystals are a unique structure created when two ions electrically attract to one another. Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. examples of ionic crystal structures. Make a rough. Ionic Crystal Examples.
From www.numerade.com
One way to describe ionic crystal structures is i… Ionic Crystal Examples Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Explain what features of a crystal are reflected in. Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Electron. Ionic Crystal Examples.
From mungfali.com
Ionic Compound Structure Ionic Crystal Examples Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. there are four types of crystals: what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Explain what features. Ionic Crystal Examples.
From courses.lumenlearning.com
Ionic Crystals Introduction to Chemistry Ionic Crystal Examples Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. examples of ionic crystal structures. Explain what features of a crystal. Ionic Crystal Examples.
From slideplayer.com
Ionic Bonds Chapter 5 Section ppt download Ionic Crystal Examples Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. Explain what features of a crystal are reflected in. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? list the properties of ionic crystals, and relate them to the lattice. Ionic Crystal Examples.
From www.slideserve.com
PPT Properties of Solids PowerPoint Presentation, free download ID Ionic Crystal Examples Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. examples of ionic crystal structures. Make a rough sketch that describes the structure. Ionic Crystal Examples.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID7053748 Ionic Crystal Examples (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. examples of ionic crystal structures. Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Make a rough sketch that describes the structure of solid sodium chloride. Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Properties and. Ionic Crystal Examples.
From www.youtube.com
Ionic Crystals YouTube Ionic Crystal Examples Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Explain what features of a crystal are reflected in. ionic crystals are a unique structure created when two ions electrically attract to one another. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Make a rough sketch that describes the structure of solid sodium chloride. . Ionic Crystal Examples.
From www.vectorstock.com
Ionic crystals the structure of sodium chloride Vector Image Ionic Crystal Examples ionic crystals are a unique structure created when two ions electrically attract to one another. Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Make a rough sketch that describes the structure of solid sodium chloride. Electron dot diagrams show the nature of the electron transfer that takes place between. Ionic Crystal Examples.
From en.wikipedia.org
Ionic compound Wikipedia Ionic Crystal Examples ionic crystals are a unique structure created when two ions electrically attract to one another. Properties and several examples of each type. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Define the lattice energy of an ionic. Ionic Crystal Examples.
From courses.lumenlearning.com
Ionic Crystals Introduction to Chemistry Ionic Crystal Examples ionic crystals are a unique structure created when two ions electrically attract to one another. Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Explain what features of a crystal are reflected in. Properties and several. Ionic Crystal Examples.
From www.graylark.com
Ionic Solids Ionic Crystal Examples examples of ionic crystal structures. there are four types of crystals: Make a rough sketch that describes the structure of solid sodium chloride. list the properties of ionic crystals, and relate them to the lattice energy. Properties and several examples of each type. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. ionic crystals are. Ionic Crystal Examples.
From learnwithdrscott.com
Ionic Bond Definition Easy Hard Science Ionic Crystal Examples list the properties of ionic crystals, and relate them to the lattice energy. Make a rough sketch that describes the structure of solid sodium chloride. Explain what features of a crystal are reflected in. there are four types of crystals: what is an ionic solid, what are its typical physical properties, and what kinds of elements does. Ionic Crystal Examples.
From www.slideserve.com
PPT Introduction to Ionic Compounds PowerPoint Presentation ID690533 Ionic Crystal Examples (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. examples of ionic crystal structures. Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. Make a rough sketch that describes the structure of solid sodium chloride. Define the lattice energy of an ionic solid in terms of the energetic properties. Ionic Crystal Examples.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Ionic Crystal Examples Explain what features of a crystal are reflected in. list the properties of ionic crystals, and relate them to the lattice energy. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Electron dot diagrams show the nature of. Ionic Crystal Examples.
From www.youtube.com
Ionic Crystal Structures {Texas A&M Intro to Materials (MSEN 201 Ionic Crystal Examples Properties and several examples of each type. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Explain what features of a crystal are reflected in. Make a rough sketch that describes the structure of solid sodium chloride. Electron dot diagrams show the nature of the electron transfer that takes. Ionic Crystal Examples.
From examples.yourdictionary.com
Ionic Crystal Examples Ionic Crystal Examples Properties and several examples of each type. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. there are four types of crystals: Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. ionic crystals are a unique structure created when two ions electrically attract to one another. Explain what. Ionic Crystal Examples.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Ionic Crystal Examples (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. list the properties of ionic crystals, and relate them to the lattice energy. Explain what features of a crystal are reflected in. Define the lattice energy of an ionic solid in terms of the energetic properties of. Ionic Crystal Examples.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID7049909 Ionic Crystal Examples what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? there are four types of crystals: Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Properties and several examples of each type. Electron dot diagrams show the nature of the. Ionic Crystal Examples.
From www.dreamstime.com
Ionic Compound, Crystal Structure with Positive and Negative Ions Ionic Crystal Examples Properties and several examples of each type. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. list the properties of ionic crystals, and relate them to the lattice energy. Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Explain what features of a crystal are reflected in. ionic. Ionic Crystal Examples.
From keystagewiki.com
Giant Ionic Structure Key Stage Wiki Ionic Crystal Examples examples of ionic crystal structures. Explain what features of a crystal are reflected in. ionic crystals are a unique structure created when two ions electrically attract to one another. there are four types of crystals: Make a rough sketch that describes the structure of solid sodium chloride. Electron dot diagrams show the nature of the electron transfer. Ionic Crystal Examples.
From www.ck12.org
Ionic Crystals Example 1 ( Video ) Chemistry CK12 Foundation Ionic Crystal Examples Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Properties and several examples of each type. list the properties of ionic crystals, and relate them to the lattice energy. examples of ionic crystal structures. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. ionic crystals are a unique structure created when two ions. Ionic Crystal Examples.
From studiousguy.com
9 Ionic Bond Examples in Daily Life StudiousGuy Ionic Crystal Examples what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Properties and several examples of each type. Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Explain what features of a crystal are reflected in. Electron dot diagrams show the nature of the electron transfer that takes. Ionic Crystal Examples.
From www.ck12.org
Ionic Crystals Overview ( Video ) Chemistry CK12 Foundation Ionic Crystal Examples there are four types of crystals: examples of ionic crystal structures. Explain what features of a crystal are reflected in. Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. ionic crystals are a unique structure created when two ions electrically attract to one another. Make a rough sketch. Ionic Crystal Examples.
From www.slideserve.com
PPT Ionic Bonds PowerPoint Presentation, free download ID1990542 Ionic Crystal Examples examples of ionic crystal structures. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. Explain what features of a crystal are reflected in. Electron dot diagrams show the nature of. Ionic Crystal Examples.
From www.slideshare.net
Giant Ionic Structure Ionic Crystal Examples Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Explain what features of a crystal are reflected in. Define the lattice energy of an ionic solid in terms of the energetic properties of its component elements. examples of ionic crystal structures. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. what is an ionic. Ionic Crystal Examples.
From www.ck12.org
Ionic Crystals Example 2 ( Video ) Chemistry CK12 Foundation Ionic Crystal Examples Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Make a rough sketch that describes the structure of solid sodium chloride. examples of ionic crystal structures. Explain what features of a crystal are reflected in. Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. (1) ionic, (2). Ionic Crystal Examples.
From www.dreamstime.com
Ionic Compound Cubic Crystal Structure Stock Vector Illustration of Ionic Crystal Examples Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain?. Ionic Crystal Examples.
From wisc.pb.unizin.org
Ionic Crystals and Unit Cell Stoichiometry (M11Q6) UWMadison Ionic Crystal Examples Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. Properties and several examples of each type. Make a rough sketch that describes the structure of solid sodium chloride. (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. . Ionic Crystal Examples.
From www.coursehero.com
Ionic Crystals Introduction to Chemistry Course Hero Ionic Crystal Examples what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Make a rough sketch that describes the structure of solid. Ionic Crystal Examples.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Ionic Crystal Examples examples of ionic crystal structures. what is an ionic solid, what are its typical physical properties, and what kinds of elements does it contain? Caesium chloride (cscl) is an ionic compound with a simple cubic lattice. Properties and several examples of each type. Make a rough sketch that describes the structure of solid sodium chloride. ionic crystals. Ionic Crystal Examples.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive Ionic Crystal Examples (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. there are four types of crystals: list the properties of ionic crystals, and relate them to the lattice energy. Properties and several examples of each type. ionic crystals are a unique structure created when two ions electrically attract to one another. Explain what features of a crystal. Ionic Crystal Examples.