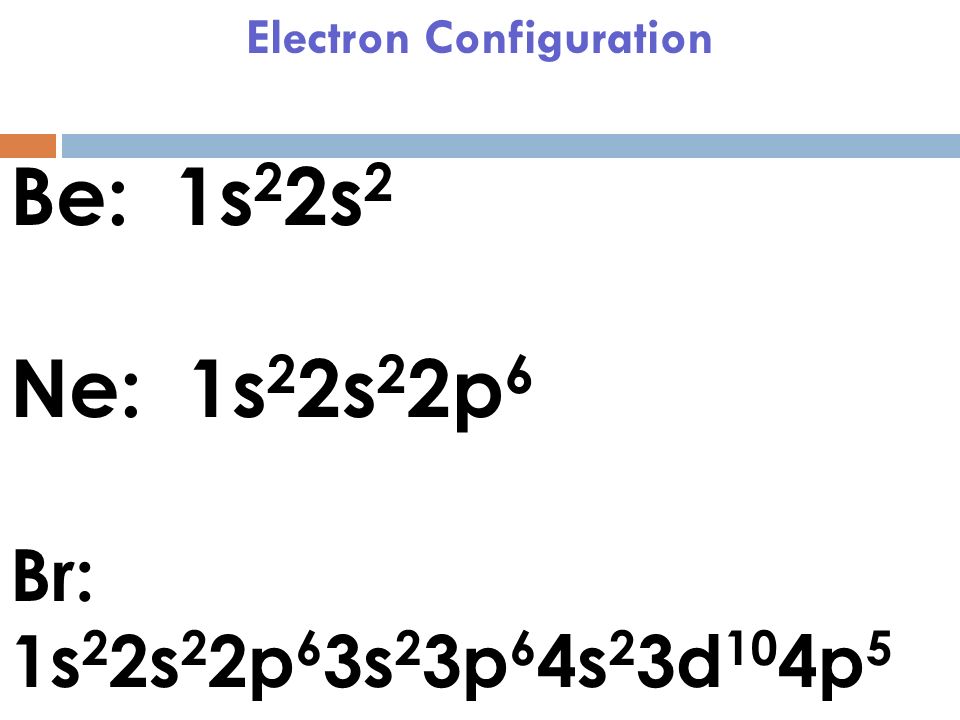
Bromine # Of Electrons . It describes how electrons are distributed among the various. In the periodic table, the elements are listed in order of increasing atomic number z. Electron configuration chart of all elements is mentioned in the table below. The atomic number of bromine represents the total number of electrons of bromine. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The shorthand electron configuration (or noble gas configuration) as well as. It has an atomic weight of 79.904 and a mass. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. It was the first element to be extracted. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. Since the atomic number of bromine is 35, the total electrons of bromine are 35.
from periodictable.me
It has an atomic weight of 79.904 and a mass. The shorthand electron configuration (or noble gas configuration) as well as. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. In the periodic table, the elements are listed in order of increasing atomic number z. Since the atomic number of bromine is 35, the total electrons of bromine are 35. It describes how electrons are distributed among the various. The atomic number of bromine represents the total number of electrons of bromine. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. It was the first element to be extracted. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals.
Bromine Electron Configuration (Br) with Orbital Diagram
Bromine # Of Electrons Electron configuration of bromine is [ar] 3d10 4s2 4p5. It was the first element to be extracted. It has an atomic weight of 79.904 and a mass. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. It describes how electrons are distributed among the various. Electron configuration chart of all elements is mentioned in the table below. Electron configuration of bromine is [ar] 3d10 4s2 4p5. The atomic number of bromine represents the total number of electrons of bromine. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. In the periodic table, the elements are listed in order of increasing atomic number z. Since the atomic number of bromine is 35, the total electrons of bromine are 35.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image & Art Alamy Bromine # Of Electrons The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The atomic number of bromine represents the total number of electrons of bromine. The shorthand electron configuration (or noble gas configuration) as well as. It was the first element to be extracted. Electron configuration chart of. Bromine # Of Electrons.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine # Of Electrons The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It was the first element to be extracted. Electron configuration chart of all elements is mentioned in the table below. Electron configuration of bromine. Bromine # Of Electrons.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Protons are represented as Bromine # Of Electrons Electron configuration chart of all elements is mentioned in the table below. Since the atomic number of bromine is 35, the total electrons of bromine are 35. It describes how electrons are distributed among the various. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. It was the first element to be extracted.. Bromine # Of Electrons.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Bromine # Of Electrons It was the first element to be extracted. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Electron configuration chart. Bromine # Of Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Bromine (Br) YouTube Bromine # Of Electrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. In the periodic table, the elements are listed in order of increasing atomic number z. Electron configuration chart of all elements is mentioned in the table below. The atomic number of bromine represents the total number of electrons of bromine.. Bromine # Of Electrons.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion Bromine # Of Electrons It has an atomic weight of 79.904 and a mass. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. It describes how electrons are distributed among the various. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number. Bromine # Of Electrons.
From www.alamy.com
Bromine molecular model Stock Vector Images Alamy Bromine # Of Electrons The shorthand electron configuration (or noble gas configuration) as well as. It has an atomic weight of 79.904 and a mass. It was the first element to be extracted. It describes how electrons are distributed among the various. Electron configuration of bromine is [ar] 3d10 4s2 4p5. The atomic number of bromine represents the total number of electrons of bromine.. Bromine # Of Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine # Of Electrons In the periodic table, the elements are listed in order of increasing atomic number z. It describes how electrons are distributed among the various. Electron configuration chart of all elements is mentioned in the table below. Since the atomic number of bromine is 35, the total electrons of bromine are 35. The shorthand electron configuration (or noble gas configuration) as. Bromine # Of Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine # Of Electrons In the periodic table, the elements are listed in order of increasing atomic number z. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number. Bromine # Of Electrons.
From www.dreamstime.com
Atom of Bromine with Detailed Core and Its 35 Electrons on Black Stock Illustration Bromine # Of Electrons Electron configuration of bromine is [ar] 3d10 4s2 4p5. It describes how electrons are distributed among the various. Since the atomic number of bromine is 35, the total electrons of bromine are 35. It has an atomic weight of 79.904 and a mass. It was the first element to be extracted. The number of electrons in each element’s electron shells,. Bromine # Of Electrons.
From app.emaze.com
Bromine ) on emaze Bromine # Of Electrons Electron configuration chart of all elements is mentioned in the table below. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. It describes how electrons are distributed among the various. Electron configuration of bromine is [ar] 3d10 4s2 4p5. In the periodic table, the elements. Bromine # Of Electrons.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Vector Image & Art Alamy Bromine # Of Electrons It was the first element to be extracted. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. It has an atomic weight of 79.904 and a mass. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Since the atomic number of bromine is 35, the total electrons of bromine are 35. It describes. Bromine # Of Electrons.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine # Of Electrons Since the atomic number of bromine is 35, the total electrons of bromine are 35. It describes how electrons are distributed among the various. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration of bromine is [ar] 3d10 4s2 4p5. The atomic number of bromine represents the total number of electrons of bromine. The number of. Bromine # Of Electrons.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Vector Image & Art Alamy Bromine # Of Electrons The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The shorthand electron configuration (or noble gas configuration) as well as. It has an atomic weight of 79.904 and a. Bromine # Of Electrons.
From www.shutterstock.com
Bohr Model Bromine Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่าลิขสิทธิ์) 1957183333 Bromine # Of Electrons The atomic number of bromine represents the total number of electrons of bromine. In the periodic table, the elements are listed in order of increasing atomic number z. It has an atomic weight of 79.904 and a mass. It was the first element to be extracted. Bromine is the 35th element in the periodic table and has a symbol of. Bromine # Of Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron configuration, and valence orbitals Bromine # Of Electrons It was the first element to be extracted. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. Since the atomic number of bromine is 35, the total electrons of bromine are 35. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its. Bromine # Of Electrons.
From www.alamy.com
Bromine molecule, illustration Stock Photo Alamy Bromine # Of Electrons The atomic number of bromine represents the total number of electrons of bromine. Since the atomic number of bromine is 35, the total electrons of bromine are 35. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It has an atomic weight of 79.904 and a mass. Electron configuration. Bromine # Of Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine # Of Electrons The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. It was the first element to be extracted. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Bromine is the 35th element in the periodic table and has a. Bromine # Of Electrons.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and comparison Periodic Table Bromine # Of Electrons The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration of bromine is [ar] 3d10 4s2 4p5. In the periodic table, the elements are listed in order of increasing atomic number z. Electron configuration chart of all elements is mentioned in the table below. Bromine is the 35th element in the periodic table and has a symbol. Bromine # Of Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine # Of Electrons It was the first element to be extracted. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Electron configuration chart of all elements is mentioned in the table below. Since the atomic number of bromine is 35, the total electrons of bromine are 35. The atomic number of bromine represents the total number of electrons of bromine. The shorthand electron. Bromine # Of Electrons.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Vector Image & Art Alamy Bromine # Of Electrons Electron configuration chart of all elements is mentioned in the table below. Since the atomic number of bromine is 35, the total electrons of bromine are 35. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The shorthand electron configuration (or noble gas configuration) as well as. It describes. Bromine # Of Electrons.
From www.alamy.com
Bromine periodic Stock Vector Images Alamy Bromine # Of Electrons It was the first element to be extracted. It has an atomic weight of 79.904 and a mass. Electron configuration chart of all elements is mentioned in the table below. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Bromine is the 35th element in. Bromine # Of Electrons.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine Bromine # Of Electrons Since the atomic number of bromine is 35, the total electrons of bromine are 35. It was the first element to be extracted. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic. Bromine # Of Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine # Of Electrons In the periodic table, the elements are listed in order of increasing atomic number z. Electron configuration chart of all elements is mentioned in the table below. The atomic number of bromine represents the total number of electrons of bromine. The shorthand electron configuration (or noble gas configuration) as well as. It was the first element to be extracted. Since. Bromine # Of Electrons.
From utedzz.blogspot.com
Bromine Periodic Table Square Periodic Table Timeline Bromine # Of Electrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It describes how electrons are distributed among the various. Since the atomic number of bromine is 35, the total electrons of bromine are 35. The atomic number of bromine represents the total number of electrons of bromine. It has an. Bromine # Of Electrons.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine # Of Electrons The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. The atomic number of bromine represents the total number of electrons of bromine. In the periodic table, the elements are listed. Bromine # Of Electrons.
From mavink.com
Lewis Dot Diagram For Bromine Bromine # Of Electrons It has an atomic weight of 79.904 and a mass. In the periodic table, the elements are listed in order of increasing atomic number z. The atomic number of bromine represents the total number of electrons of bromine. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical. Bromine # Of Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine # Of Electrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The shorthand electron configuration (or noble gas configuration) as well as. It has an atomic weight of 79.904 and a mass. Since the atomic number of bromine is 35, the total electrons of bromine are 35. The electron configuration of. Bromine # Of Electrons.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine # Of Electrons The atomic number of bromine represents the total number of electrons of bromine. It describes how electrons are distributed among the various. In the periodic table, the elements are listed in order of increasing atomic number z. The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. Electron configuration of bromine is [ar] 3d10. Bromine # Of Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine # Of Electrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Electron configuration chart of all elements is mentioned in the table below. Electron configuration of bromine is [ar] 3d10 4s2 4p5. It describes how electrons are distributed among the various. The electron configuration of bromine refers to the arrangement of. Bromine # Of Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron configuration, chemical data, and Bromine # Of Electrons Electron configuration of bromine is [ar] 3d10 4s2 4p5. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It describes how electrons are distributed among the various. The shorthand electron configuration (or noble gas configuration) as well as. Since the atomic number of bromine is 35, the total electrons. Bromine # Of Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine # Of Electrons The shorthand electron configuration (or noble gas configuration) as well as. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Since the atomic number of bromine is 35, the total electrons of bromine are 35. It has an atomic weight of 79.904 and a mass. Electron configuration chart of. Bromine # Of Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine # Of Electrons Electron configuration chart of all elements is mentioned in the table below. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It describes how electrons are distributed among the various. Electron configuration of bromine is [ar] 3d10 4s2 4p5. It has an atomic weight of 79.904 and a mass.. Bromine # Of Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine # Of Electrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. In the periodic table, the elements are listed in order of increasing atomic number z. It has an atomic weight of 79.904 and a mass. It was the first element to be extracted. It describes how electrons are distributed among. Bromine # Of Electrons.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Bromine # Of Electrons The electron configuration of bromine refers to the arrangement of electrons in the bromine atom’s orbitals. It was the first element to be extracted. It describes how electrons are distributed among the various. Electron configuration of bromine is [ar] 3d10 4s2 4p5. Electron configuration chart of all elements is mentioned in the table below. Bromine is the 35th element in. Bromine # Of Electrons.