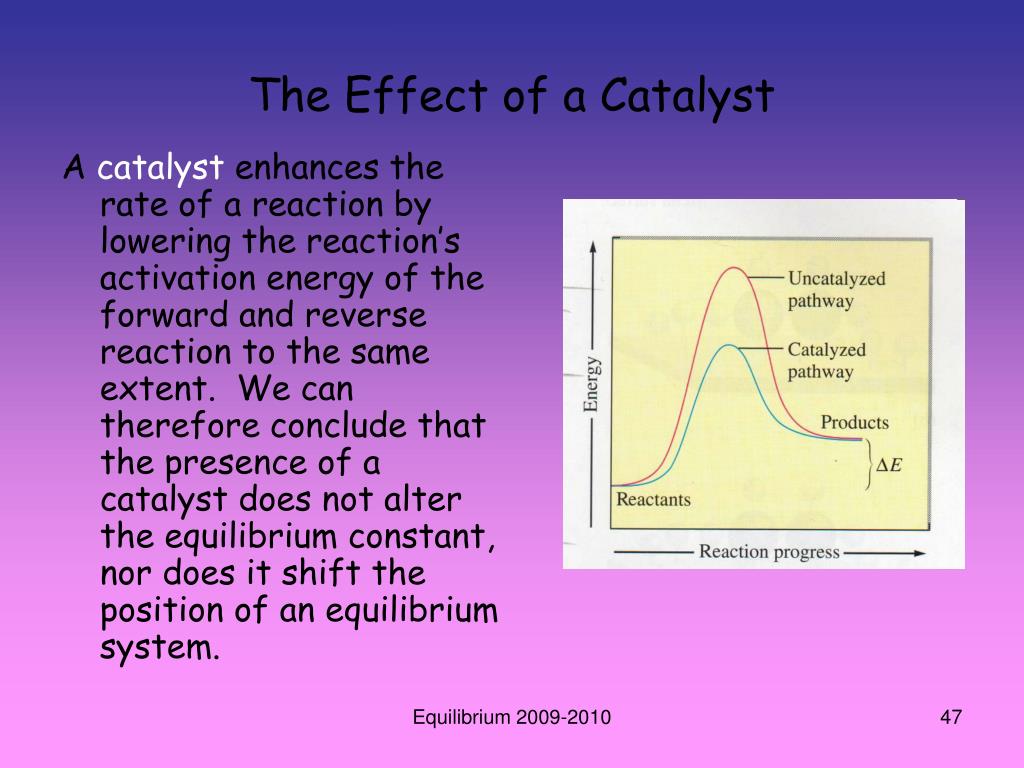
What Does Adding A Catalyst Do To Equilibrium . This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. The effect of adding a catalyst on an equilibrium. It covers changes to the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. The effect of a catalyst on equilibrium. Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. It does however allow equilibrium. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature.
from www.slideserve.com
This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. The effect of a catalyst on equilibrium. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. It does however allow equilibrium. The effect of adding a catalyst on an equilibrium. It covers changes to the.
PPT Chemical Equilibrium PowerPoint Presentation, free download ID5967535
What Does Adding A Catalyst Do To Equilibrium Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. It does however allow equilibrium. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. It covers changes to the. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. The effect of adding a catalyst on an equilibrium. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. The effect of a catalyst on equilibrium. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst What Does Adding A Catalyst Do To Equilibrium Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. The effect of adding a catalyst on an equilibrium. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. It. What Does Adding A Catalyst Do To Equilibrium.
From slideplayer.com
Factors That Affect Equilibrium ppt download What Does Adding A Catalyst Do To Equilibrium It covers changes to the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. The effect of adding a catalyst on an equilibrium. Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change.. What Does Adding A Catalyst Do To Equilibrium.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 What Does Adding A Catalyst Do To Equilibrium It covers changes to the. It does however allow equilibrium. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. Adding a chemical that is present on either side. What Does Adding A Catalyst Do To Equilibrium.
From www.slideserve.com
PPT Le Chatelier's Principle PowerPoint Presentation, free download ID4070791 What Does Adding A Catalyst Do To Equilibrium This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. It does however allow equilibrium. The effect of adding a catalyst on an equilibrium.. What Does Adding A Catalyst Do To Equilibrium.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent What Does Adding A Catalyst Do To Equilibrium It does however allow equilibrium. The effect of adding a catalyst on an equilibrium. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. This brief page explains why adding a ctalyst to an. What Does Adding A Catalyst Do To Equilibrium.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent What Does Adding A Catalyst Do To Equilibrium Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. The effect of a catalyst on equilibrium. It covers changes to the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. If a. What Does Adding A Catalyst Do To Equilibrium.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free download ID6591070 What Does Adding A Catalyst Do To Equilibrium If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent. What Does Adding A Catalyst Do To Equilibrium.
From 2012books.lardbucket.org
Catalysis What Does Adding A Catalyst Do To Equilibrium The effect of a catalyst on equilibrium. Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of. What Does Adding A Catalyst Do To Equilibrium.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) What Does Adding A Catalyst Do To Equilibrium Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. It does however allow equilibrium. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. This page. What Does Adding A Catalyst Do To Equilibrium.
From www.youtube.com
Disturbing equilibrium Effect of adding a catalyst on chemical equilibrium YouTube What Does Adding A Catalyst Do To Equilibrium It covers changes to the. The effect of adding a catalyst on an equilibrium. It does however allow equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. If we have a system. What Does Adding A Catalyst Do To Equilibrium.
From www.slideserve.com
PPT Rates of Reaction & Equilibrium PowerPoint Presentation, free download ID5783086 What Does Adding A Catalyst Do To Equilibrium This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. The effect of adding a catalyst on an equilibrium. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. If a catalyst is added. What Does Adding A Catalyst Do To Equilibrium.
From courses.lumenlearning.com
Catalysis Chemistry What Does Adding A Catalyst Do To Equilibrium Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. It covers changes to the. Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as. What Does Adding A Catalyst Do To Equilibrium.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review PowerPoint Presentation ID6055094 What Does Adding A Catalyst Do To Equilibrium It does however allow equilibrium. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. If we have a system which is already in equilibrium, addition of an extra amount of one of. What Does Adding A Catalyst Do To Equilibrium.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 What Does Adding A Catalyst Do To Equilibrium It covers changes to the. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. The effect of adding a catalyst on an equilibrium. The effect of a catalyst on equilibrium. If we. What Does Adding A Catalyst Do To Equilibrium.
From www.waca.msf.org
Catalyst, Catalyst What Does Adding A Catalyst Do To Equilibrium This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. Adding a chemical that is present on either side of the equation will cause a shift in the position. What Does Adding A Catalyst Do To Equilibrium.
From www.youtube.com
Effect of Catalyst on Equilibrium Constant, Chemistry Lecture Sabaq.pk YouTube What Does Adding A Catalyst Do To Equilibrium This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. It does however allow equilibrium. It covers changes to the. In the case of temperature, the value of. What Does Adding A Catalyst Do To Equilibrium.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts What Does Adding A Catalyst Do To Equilibrium The effect of a catalyst on equilibrium. It does however allow equilibrium. The effect of adding a catalyst on an equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. It covers changes to the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. This page. What Does Adding A Catalyst Do To Equilibrium.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and reversible reactions What Does Adding A Catalyst Do To Equilibrium The effect of adding a catalyst on an equilibrium. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on. What Does Adding A Catalyst Do To Equilibrium.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram What Does Adding A Catalyst Do To Equilibrium Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. The effect of adding a catalyst on an equilibrium. If a catalyst is added to a reaction, both the. What Does Adding A Catalyst Do To Equilibrium.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis What Does Adding A Catalyst Do To Equilibrium The effect of a catalyst on equilibrium. The effect of adding a catalyst on an equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. This page looks at le chatelier's principle and explains how to. What Does Adding A Catalyst Do To Equilibrium.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst What Does Adding A Catalyst Do To Equilibrium It covers changes to the. The effect of a catalyst on equilibrium. The effect of adding a catalyst on an equilibrium. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. In the case of temperature, the. What Does Adding A Catalyst Do To Equilibrium.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps What Does Adding A Catalyst Do To Equilibrium The effect of adding a catalyst on an equilibrium. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. The effect of a catalyst on equilibrium. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on. What Does Adding A Catalyst Do To Equilibrium.
From slideplayer.com
Chapter 14 Chemical Equilibrium ppt download What Does Adding A Catalyst Do To Equilibrium It covers changes to the. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. This brief page explains why adding a ctalyst to an equilibrium reaction has no. What Does Adding A Catalyst Do To Equilibrium.
From ar.inspiredpencil.com
Chemical Equilibrium What Does Adding A Catalyst Do To Equilibrium Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. If a catalyst is added to a reaction, both the forward and reverse. What Does Adding A Catalyst Do To Equilibrium.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Presentation ID1132028 What Does Adding A Catalyst Do To Equilibrium This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. In the case of temperature, the value of the equilibrium has. What Does Adding A Catalyst Do To Equilibrium.
From h-o-m-e.org
Catalyst Tipping the Scales of Equilibrium What Does Adding A Catalyst Do To Equilibrium If a catalyst is added to a reaction, both the forward and reverse reaction rates will. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to. What Does Adding A Catalyst Do To Equilibrium.
From www.youtube.com
Equilibrium Graphs Le Chatelier's Principle (Chemical Equilibrium). YouTube What Does Adding A Catalyst Do To Equilibrium Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. This page looks at le chatelier's principle and explains how to apply it to. What Does Adding A Catalyst Do To Equilibrium.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.21C Understand Why a Catalyst Does Not Affect the Position of What Does Adding A Catalyst Do To Equilibrium This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. It covers changes to the. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. This page looks at le chatelier's principle and explains how to apply it to reactions in a state. What Does Adding A Catalyst Do To Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID5967535 What Does Adding A Catalyst Do To Equilibrium It does however allow equilibrium. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. The effect of a catalyst on equilibrium. It covers changes to the. If a. What Does Adding A Catalyst Do To Equilibrium.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent What Does Adding A Catalyst Do To Equilibrium The effect of adding a catalyst on an equilibrium. If a catalyst is added to a reaction, both the forward and reverse reaction rates will. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. In the case of temperature, the value of the equilibrium has changed because the k eq is dependent. What Does Adding A Catalyst Do To Equilibrium.
From www.youtube.com
Equilibrium Graphs grade 12 Catalyst YouTube What Does Adding A Catalyst Do To Equilibrium If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. The effect of a catalyst on equilibrium. It covers changes to the. It does however allow equilibrium. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. If a. What Does Adding A Catalyst Do To Equilibrium.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 What Does Adding A Catalyst Do To Equilibrium Adding a chemical that is present on either side of the equation will cause a shift in the position of the equilibrium, as the system adjusts to counteract the change. It covers changes to the. Adding a catalyst to a reaction at equilibrium has no effect on the position of equilibrium. This brief page explains why adding a ctalyst to. What Does Adding A Catalyst Do To Equilibrium.
From www.youtube.com
7.1 The effect of a catalyst on an equilibrium reaction (SL) YouTube What Does Adding A Catalyst Do To Equilibrium In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. The effect of adding a catalyst on an equilibrium. It covers changes to the. This page looks at le chatelier's principle and explains. What Does Adding A Catalyst Do To Equilibrium.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts What Does Adding A Catalyst Do To Equilibrium This brief page explains why adding a ctalyst to an equilibrium reaction has no effect on the. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. The effect of adding a catalyst on an equilibrium. The effect of a catalyst on equilibrium. It does however allow equilibrium. Adding. What Does Adding A Catalyst Do To Equilibrium.
From www.savemyexams.com
The Position of Equilibrium Edexcel IGCSE Chemistry Revision Notes 2019 What Does Adding A Catalyst Do To Equilibrium In the case of temperature, the value of the equilibrium has changed because the k eq is dependent on temperature. It covers changes to the. The effect of adding a catalyst on an equilibrium. If we have a system which is already in equilibrium, addition of an extra amount of one of the reactants or one of the products. The. What Does Adding A Catalyst Do To Equilibrium.