Standard Enthalpy Of Formation Triiodide . The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their.
from www.chegg.com
Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the.
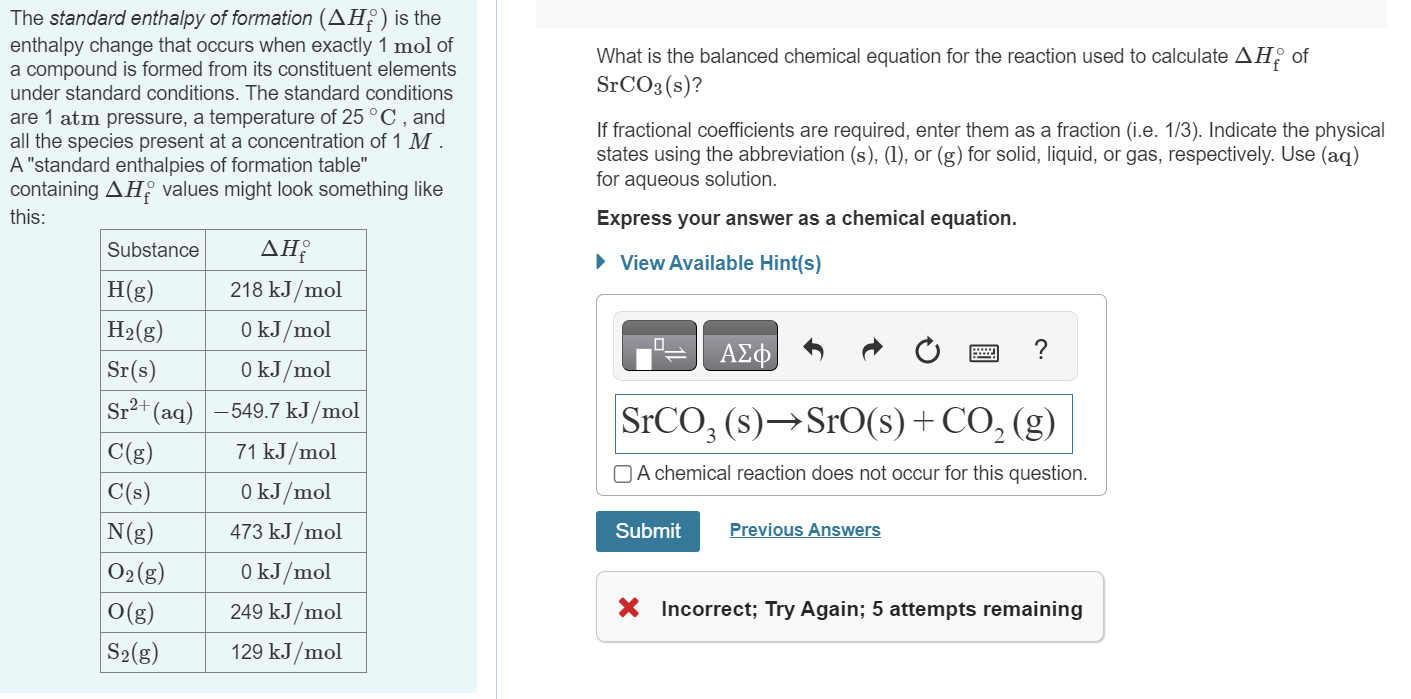
Solved The standard enthalpy of formation (ΔHf∘) is the
Standard Enthalpy Of Formation Triiodide Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. The standard enthalpy of formation of any element in its standard state is zero by definition.
From www.youtube.com
Standard Enthalpy of Formation YouTube Standard Enthalpy Of Formation Triiodide Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standard enthalpy of formation. Standard Enthalpy Of Formation Triiodide.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Triiodide Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This version of atct results [3] was generated by additional expansion of version 1.148. Standard Enthalpy Of Formation Triiodide.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID6642303 Standard Enthalpy Of Formation Triiodide This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Selected. Standard Enthalpy Of Formation Triiodide.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation Triiodide For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. This version. Standard Enthalpy Of Formation Triiodide.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Triiodide Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy. Standard Enthalpy Of Formation Triiodide.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Triiodide The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in. Standard Enthalpy Of Formation Triiodide.
From www.studypool.com
SOLUTION Standard enthalpy of formation Studypool Standard Enthalpy Of Formation Triiodide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard. Standard Enthalpy Of Formation Triiodide.
From www.chegg.com
Solved The standard enthalpy of formation (ΔHf∘) is the Standard Enthalpy Of Formation Triiodide The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. This version. Standard Enthalpy Of Formation Triiodide.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Triiodide Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Triiodide.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation* for Various Compounds Standard Enthalpy Of Formation Triiodide This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard. Standard Enthalpy Of Formation Triiodide.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Triiodide Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. 193. Standard Enthalpy Of Formation Triiodide.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5072129 Standard Enthalpy Of Formation Triiodide Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of. Standard Enthalpy Of Formation Triiodide.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Triiodide Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole. Standard Enthalpy Of Formation Triiodide.
From www.researchgate.net
Molecular weight, standard enthalpy of formation and thermal... Download Scientific Diagram Standard Enthalpy Of Formation Triiodide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. Standard Enthalpy Of Formation Triiodide.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work... Download Table Standard Enthalpy Of Formation Triiodide Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Enthalpy Of Formation Triiodide.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Enthalpy Of Formation Triiodide For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. Standard Enthalpy Of Formation Triiodide.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Triiodide Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. This version of. Standard Enthalpy Of Formation Triiodide.
From www.slideserve.com
PPT Chemical Thermodynamics PowerPoint Presentation, free download ID5375335 Standard Enthalpy Of Formation Triiodide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network. Standard Enthalpy Of Formation Triiodide.
From mungfali.com
Enthalpies Of Formation Table Standard Enthalpy Of Formation Triiodide For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. The standard enthalpy. Standard Enthalpy Of Formation Triiodide.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Triiodide Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation Triiodide.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Triiodide Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole. Standard Enthalpy Of Formation Triiodide.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Chemistry 2e 5.3 YouTube Standard Enthalpy Of Formation Triiodide Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Enthalpy Of Formation Triiodide.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Triiodide The standard enthalpy of formation of any element in its standard state is zero by definition. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy. Standard Enthalpy Of Formation Triiodide.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Triiodide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this. Standard Enthalpy Of Formation Triiodide.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Appendix I to calculate the Standard Enthalpy Of Formation Triiodide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of. Standard Enthalpy Of Formation Triiodide.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Triiodide The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standard enthalpy. Standard Enthalpy Of Formation Triiodide.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free download ID4820880 Standard Enthalpy Of Formation Triiodide The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. This. Standard Enthalpy Of Formation Triiodide.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Triiodide The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of. Standard Enthalpy Of Formation Triiodide.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Triiodide Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a. Standard Enthalpy Of Formation Triiodide.
From www.researchgate.net
The calculated M index and the experimental standard enthalpy of... Download Table Standard Enthalpy Of Formation Triiodide For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This version. Standard Enthalpy Of Formation Triiodide.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol ) ( ) g 0 223 aq 56 aq 182 s Standard Enthalpy Of Formation Triiodide This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the. Standard Enthalpy Of Formation Triiodide.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Triiodide This version of atct results [3] was generated by additional expansion of version 1.148 to include species relevant to a recent study of the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic. Standard Enthalpy Of Formation Triiodide.
From www.chegg.com
Solved Use the standard enthalpies of formation from your Standard Enthalpy Of Formation Triiodide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The standard enthalpy of formation is a measure of the energy released or consumed when. Standard Enthalpy Of Formation Triiodide.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube Standard Enthalpy Of Formation Triiodide Standard enthalpy of formation (δhf°) represents the enthalpy change when one mole of a compound is formed from its elements in their. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy. Standard Enthalpy Of Formation Triiodide.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Enthalpy Of Formation Triiodide Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Standard Enthalpy Of Formation Triiodide.