Does Salt Dissolve In Acid . You want to know how can we predict it just by. In summary, some salts do not react with acids due to their solubility in water or other factors. This is due either to the presence. However, certain salts can still. Salts with a hydrolyzable cation. Apr 7, 2016 at 20:41. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. How is that more soluble than cahpo4? Determine if a salt produces an acidic or a basic solution. Except for their names and formulas, so far we have treated all acids as equals,. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid.
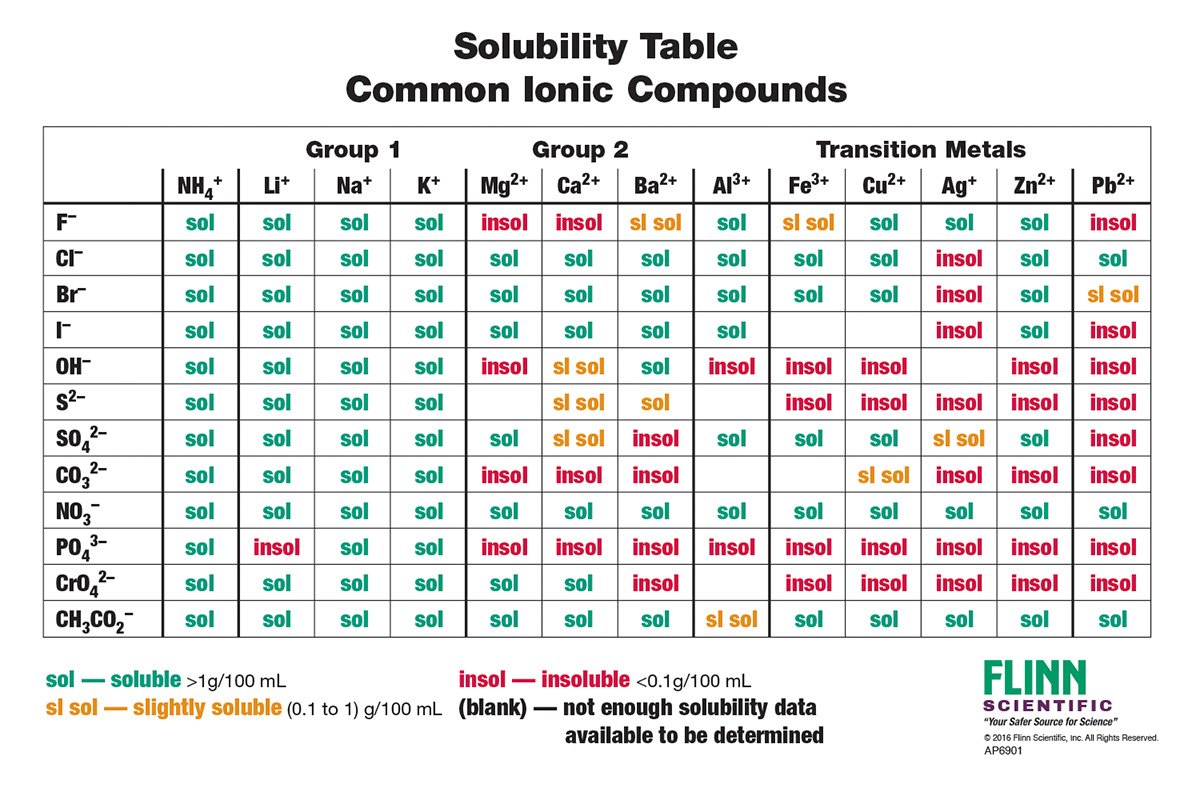
from www.flinnsci.ca
This is due either to the presence. However, certain salts can still. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Apr 7, 2016 at 20:41. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. In summary, some salts do not react with acids due to their solubility in water or other factors. Salts with a hydrolyzable cation. How is that more soluble than cahpo4? You want to know how can we predict it just by. Except for their names and formulas, so far we have treated all acids as equals,.
Solubility Rules Chart, Notebook Size, Pad of 30 Flinn Scientific
Does Salt Dissolve In Acid When dissolved in water, acidic salts will yield solutions with ph less than 7.0. However, certain salts can still. Salts with a hydrolyzable cation. How is that more soluble than cahpo4? A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. You want to know how can we predict it just by. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Apr 7, 2016 at 20:41. This is due either to the presence. Determine if a salt produces an acidic or a basic solution. Except for their names and formulas, so far we have treated all acids as equals,. In summary, some salts do not react with acids due to their solubility in water or other factors.
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow Does Salt Dissolve In Acid A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. How is that more soluble than cahpo4? When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Except for their names and formulas, so far we have treated. Does Salt Dissolve In Acid.
From ar.inspiredpencil.com
Solubility Science Does Salt Dissolve In Acid Except for their names and formulas, so far we have treated all acids as equals,. Apr 7, 2016 at 20:41. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. In summary, some salts do not react with acids due to their solubility in water or other factors. How is that more soluble than cahpo4? This. Does Salt Dissolve In Acid.
From 2012books.lardbucket.org
Solutions Does Salt Dissolve In Acid How is that more soluble than cahpo4? Determine if a salt produces an acidic or a basic solution. You want to know how can we predict it just by. However, certain salts can still. Except for their names and formulas, so far we have treated all acids as equals,. When dissolved in water, acidic salts will yield solutions with ph. Does Salt Dissolve In Acid.
From www.pinterest.com
saturated unsaturated and supersaturated solutions Google Search Does Salt Dissolve In Acid Salts with a hydrolyzable cation. You want to know how can we predict it just by. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. This is due either to the presence. Apr 7, 2016 at 20:41. Except for their names and formulas, so far we have treated all acids as equals,. Determine if a. Does Salt Dissolve In Acid.
From www.youtube.com
Why Salt (NaCl) Does Not Dissolve in Oil Salt and Oil Experiment Is Does Salt Dissolve In Acid In summary, some salts do not react with acids due to their solubility in water or other factors. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. How is that more soluble than cahpo4? You want to know how can we. Does Salt Dissolve In Acid.
From www.reddit.com
Spontaneous Reactions and solids that will dissolve in acids r/chemhelp Does Salt Dissolve In Acid In summary, some salts do not react with acids due to their solubility in water or other factors. Salts with a hydrolyzable cation. Determine if a salt produces an acidic or a basic solution. You want to know how can we predict it just by. A salt can dissolve in water to produce a neutral, a basic, or an acidic. Does Salt Dissolve In Acid.
From www.youtube.com
How does water dissolve salt? YouTube Does Salt Dissolve In Acid Except for their names and formulas, so far we have treated all acids as equals,. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Apr 7, 2016 at 20:41. How is that more soluble than cahpo4? However, certain salts can still.. Does Salt Dissolve In Acid.
From 88guru.com
What are Salts in Chemistry Characteristics, Types 88guru Does Salt Dissolve In Acid How is that more soluble than cahpo4? Salts with a hydrolyzable cation. Except for their names and formulas, so far we have treated all acids as equals,. Apr 7, 2016 at 20:41. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. A salt can dissolve in water to produce a neutral, a basic, or an. Does Salt Dissolve In Acid.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Does Salt Dissolve In Acid However, certain salts can still. Determine if a salt produces an acidic or a basic solution. You want to know how can we predict it just by. In summary, some salts do not react with acids due to their solubility in water or other factors. This is due either to the presence. Apr 7, 2016 at 20:41. A salt can. Does Salt Dissolve In Acid.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Does Salt Dissolve In Acid A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. You want to know how can we predict it just by. How is that more soluble than cahpo4? However, certain salts can still. Apr 7, 2016 at 20:41. Except for their names. Does Salt Dissolve In Acid.
From elchoroukhost.net
Write The Chemical Formulas Of Two Ions When Table Salt Dissolved In Does Salt Dissolve In Acid You want to know how can we predict it just by. In summary, some salts do not react with acids due to their solubility in water or other factors. Determine if a salt produces an acidic or a basic solution. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it. Does Salt Dissolve In Acid.
From www.youtube.com
Why Does Water Dissolve Sugar? YouTube Does Salt Dissolve In Acid In summary, some salts do not react with acids due to their solubility in water or other factors. How is that more soluble than cahpo4? Determine if a salt produces an acidic or a basic solution. Except for their names and formulas, so far we have treated all acids as equals,. Salts with a hydrolyzable cation. This is due either. Does Salt Dissolve In Acid.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Does Salt Dissolve In Acid A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Apr 7, 2016 at 20:41. In summary, some salts do not react with acids due to their solubility. Does Salt Dissolve In Acid.
From www.pinterest.jp
Solubility Rules Chart for Chemistry Classroom Teaching chemistry Does Salt Dissolve In Acid However, certain salts can still. Apr 7, 2016 at 20:41. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Salts with a hydrolyzable cation. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Determine if a. Does Salt Dissolve In Acid.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Does Salt Dissolve In Acid You want to know how can we predict it just by. Apr 7, 2016 at 20:41. However, certain salts can still. Determine if a salt produces an acidic or a basic solution. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. A salt can dissolve in water to produce a neutral, a basic, or an. Does Salt Dissolve In Acid.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Does Salt Dissolve In Acid Except for their names and formulas, so far we have treated all acids as equals,. However, certain salts can still. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. In summary, some salts do not react with acids due to their. Does Salt Dissolve In Acid.
From www.thoughtco.com
What Is the CommonIon Effect? Does Salt Dissolve In Acid This is due either to the presence. Apr 7, 2016 at 20:41. You want to know how can we predict it just by. Salts with a hydrolyzable cation. In summary, some salts do not react with acids due to their solubility in water or other factors. How is that more soluble than cahpo4? However, certain salts can still. Determine if. Does Salt Dissolve In Acid.
From www.docsity.com
Solubility General Chemistry Lecture Slides Docsity Does Salt Dissolve In Acid You want to know how can we predict it just by. This is due either to the presence. How is that more soluble than cahpo4? In summary, some salts do not react with acids due to their solubility in water or other factors. However, certain salts can still. Salts with a hydrolyzable cation. A salt can dissolve in water to. Does Salt Dissolve In Acid.
From www.animalia-life.club
Solubility Rules Zumdahl Does Salt Dissolve In Acid When dissolved in water, acidic salts will yield solutions with ph less than 7.0. In summary, some salts do not react with acids due to their solubility in water or other factors. You want to know how can we predict it just by. Except for their names and formulas, so far we have treated all acids as equals,. However, certain. Does Salt Dissolve In Acid.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Does Salt Dissolve In Acid Except for their names and formulas, so far we have treated all acids as equals,. You want to know how can we predict it just by. Salts with a hydrolyzable cation. Apr 7, 2016 at 20:41. How is that more soluble than cahpo4? A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending. Does Salt Dissolve In Acid.
From dissolvesalt.weebly.com
scientific report does salt dissolve quicker in hot or cold water? Does Salt Dissolve In Acid When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Salts with a hydrolyzable cation. You want to know how can we predict it just by. However, certain salts can still. Except for their names and formulas, so far we have treated all acids as equals,. A salt can dissolve in water to produce a neutral,. Does Salt Dissolve In Acid.
From www.numerade.com
SOLVED when salt is dissolved in water why cant the individual Does Salt Dissolve In Acid You want to know how can we predict it just by. Apr 7, 2016 at 20:41. How is that more soluble than cahpo4? Except for their names and formulas, so far we have treated all acids as equals,. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Salts with a hydrolyzable cation. This is due. Does Salt Dissolve In Acid.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Does Salt Dissolve In Acid You want to know how can we predict it just by. Determine if a salt produces an acidic or a basic solution. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. This is due either to the presence. When dissolved in. Does Salt Dissolve In Acid.
From www.youtube.com
Why Salt Dissolve In Water/உப்பு ஏன் தண்ணீரில் கரைகிறது YouTube Does Salt Dissolve In Acid When dissolved in water, acidic salts will yield solutions with ph less than 7.0. In summary, some salts do not react with acids due to their solubility in water or other factors. How is that more soluble than cahpo4? You want to know how can we predict it just by. Determine if a salt produces an acidic or a basic. Does Salt Dissolve In Acid.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube Does Salt Dissolve In Acid Determine if a salt produces an acidic or a basic solution. This is due either to the presence. Except for their names and formulas, so far we have treated all acids as equals,. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Salts with a hydrolyzable cation. A salt can dissolve in water to produce. Does Salt Dissolve In Acid.
From sciencenotes.org
Is Dissolving Salt in Water a Chemical Change or a Physical Change? Does Salt Dissolve In Acid You want to know how can we predict it just by. How is that more soluble than cahpo4? This is due either to the presence. In summary, some salts do not react with acids due to their solubility in water or other factors. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Apr 7, 2016. Does Salt Dissolve In Acid.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.6.1 Solubility Rules Does Salt Dissolve In Acid Apr 7, 2016 at 20:41. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. In summary, some salts do not react with acids due to their solubility in water or other factors. Determine if a salt produces an acidic or a basic solution. This is due either to the presence. Except for their names and. Does Salt Dissolve In Acid.
From communitystudentlearning.org
What Does Oil Dissolve In What Dissolves Oil? Does Salt Dissolve In Acid You want to know how can we predict it just by. Except for their names and formulas, so far we have treated all acids as equals,. How is that more soluble than cahpo4? When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Salts with a hydrolyzable cation. Determine if a salt produces an acidic or. Does Salt Dissolve In Acid.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Does Salt Dissolve In Acid How is that more soluble than cahpo4? A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Apr 7, 2016 at 20:41. You want to know how can we predict it just by. However, certain salts can still. This is due either. Does Salt Dissolve In Acid.
From powerupcook.com
How Does Salt Dissolve In Water Power Up Cook Does Salt Dissolve In Acid A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. You want to know how can we predict it just by. In summary, some salts do not react with acids due to their solubility in water or other factors. However, certain salts. Does Salt Dissolve In Acid.
From www.youtube.com
Dissolution (chemistry) YouTube Does Salt Dissolve In Acid When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Salts with a hydrolyzable cation. How is that more soluble than cahpo4? Except for their names and formulas, so far we have treated all acids as equals,. You want to know how can we predict it just by. Apr 7, 2016 at 20:41. This is due. Does Salt Dissolve In Acid.
From www.flinnsci.ca
Solubility Rules Chart, Notebook Size, Pad of 30 Flinn Scientific Does Salt Dissolve In Acid Determine if a salt produces an acidic or a basic solution. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. Except for their names and formulas, so. Does Salt Dissolve In Acid.
From www.youtube.com
Acidbase properties of salts Acids and bases AP Chemistry Khan Does Salt Dissolve In Acid How is that more soluble than cahpo4? Except for their names and formulas, so far we have treated all acids as equals,. Salts with a hydrolyzable cation. When dissolved in water, acidic salts will yield solutions with ph less than 7.0. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether. Does Salt Dissolve In Acid.
From faculty.uml.edu
Untitled [faculty.uml.edu] Does Salt Dissolve In Acid However, certain salts can still. Apr 7, 2016 at 20:41. This is due either to the presence. Salts with a hydrolyzable cation. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Determine if a salt produces an acidic or a basic. Does Salt Dissolve In Acid.
From www.youtube.com
Solubility of Salts Acid, Base and Salt YouTube Does Salt Dissolve In Acid In summary, some salts do not react with acids due to their solubility in water or other factors. You want to know how can we predict it just by. Salts with a hydrolyzable cation. However, certain salts can still. Except for their names and formulas, so far we have treated all acids as equals,. Determine if a salt produces an. Does Salt Dissolve In Acid.