Examples Of Gases Soluble In Water . Bringing a liquid to its boiling point will completely remove a gaseous solute. The solubility of gases in liquid is affected by pressure and temperature. Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. At 20 °c, the concentration of dissolved oxygen in water. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1.
from www.slideserve.com
Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. At 20 °c, the concentration of dissolved oxygen in water. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. The solubility of gases in liquid is affected by pressure and temperature. Bringing a liquid to its boiling point will completely remove a gaseous solute. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,.
PPT Solutions PowerPoint Presentation, free download ID1820539
Examples Of Gases Soluble In Water At 20 °c, the concentration of dissolved oxygen in water. Bringing a liquid to its boiling point will completely remove a gaseous solute. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. At 20 °c, the concentration of dissolved oxygen in water. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. The solubility of gases in liquid is affected by pressure and temperature.
From www.slideserve.com
PPT Chapter 9 Solutions PowerPoint Presentation, free download ID Examples Of Gases Soluble In Water At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. Examples of gases that dissolve in water are carbon dioxide,. Examples Of Gases Soluble In Water.
From www.scienceabc.com
Why Do Bubbles Form In A Glass Of Water That's Left Out? » ScienceABC Examples Of Gases Soluble In Water At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Bringing a liquid to its boiling point will completely remove a gaseous solute.. Examples Of Gases Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Examples Of Gases Soluble In Water Bringing a liquid to its boiling point will completely remove a gaseous solute. The solubility of gases in liquid is affected by pressure and temperature. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID3105812 Examples Of Gases Soluble In Water At 20 °c, the concentration of dissolved oxygen in water. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. The solubility of gases in liquid is affected by pressure. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Gases in Solution PowerPoint Presentation, free download ID5701906 Examples Of Gases Soluble In Water Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. Bringing a liquid to its boiling point will completely remove a gaseous solute. At 20 °c, the concentration of dissolved oxygen in water. The solubility of gases in liquid is affected by pressure and. Examples Of Gases Soluble In Water.
From www.doubtnut.com
Solubility of oxygen gas in water follows Henry's law. When the solubi Examples Of Gases Soluble In Water Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. The solubility of gases in liquid is affected by pressure and temperature. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Gases in Solution PowerPoint Presentation, free download ID5701906 Examples Of Gases Soluble In Water At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. At 20 °c, the concentration of dissolved oxygen in water. Bringing a liquid to its boiling point. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT The solubility of gases in water usually decreases with Examples Of Gases Soluble In Water Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. At 20 °c, the concentration of dissolved oxygen in water. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. Bringing a liquid to its boiling point will completely remove a gaseous solute. Understanding the. Examples Of Gases Soluble In Water.
From methanegaskimawashi.blogspot.com
Methane Gas Solubility Of Methane Gas In Water Examples Of Gases Soluble In Water Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. The solubility of gases in liquid is affected by pressure and temperature. Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous. Examples Of Gases Soluble In Water.
From www.elgalabwater.com
Dissolved Gases in Purified Water ELGA LabWater Examples Of Gases Soluble In Water At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. Some typical gas solubilities, expressed. Examples Of Gases Soluble In Water.
From courses.lumenlearning.com
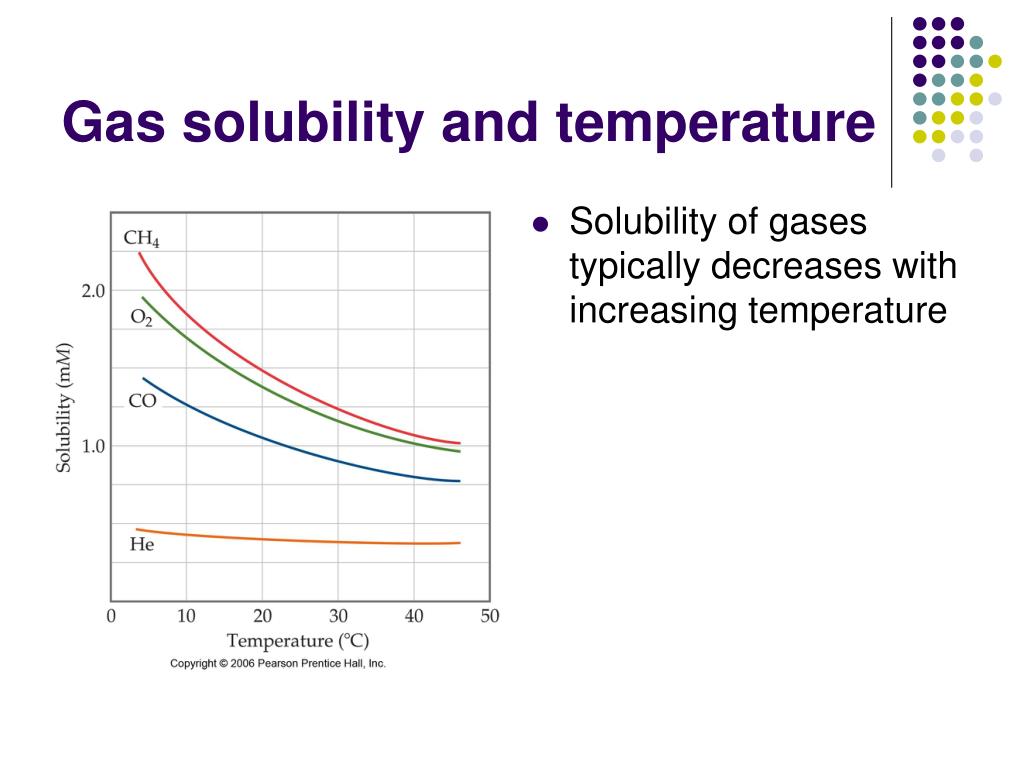
Gas Solubility and Temperature Introduction to Chemistry Examples Of Gases Soluble In Water Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. The solubility of gases in liquid is affected by pressure and temperature. Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. At 20 °c, the concentration of dissolved oxygen in. Examples Of Gases Soluble In Water.
From slideplayer.com
Principles of Chemistry A Molecular Approach, 1st Ed. Nivaldo Tro Examples Of Gases Soluble In Water Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. Bringing a liquid to its boiling point will completely remove a gaseous solute. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Examples of gases that dissolve in water are. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Examples Of Gases Soluble In Water At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. At 20 °c, the concentration. Examples Of Gases Soluble In Water.
From www.researchgate.net
Gas solubility data in pure water. Download Table Examples Of Gases Soluble In Water Bringing a liquid to its boiling point will completely remove a gaseous solute. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. At 20 °c, the concentration of dissolved oxygen in water. Understanding the water solubility of. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Ocean Water Chemistry PowerPoint Presentation, free download ID Examples Of Gases Soluble In Water At 20 °c, the concentration of dissolved oxygen in water. The solubility of gases in liquid is affected by pressure and temperature. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. Solubilities given for those gases which react with water, namely ozone, nitrogen. Examples Of Gases Soluble In Water.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Examples Of Gases Soluble In Water The solubility of gases in liquid is affected by pressure and temperature. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. At 20 °c, the concentration of dissolved oxygen. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Ch 15/16 Water and its Properties. Test Review PowerPoint Examples Of Gases Soluble In Water Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a. Examples Of Gases Soluble In Water.
From methanegaskimawashi.blogspot.com
Methane Gas Solubility Of Methane Gas In Water Examples Of Gases Soluble In Water Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. At 20. Examples Of Gases Soluble In Water.
From www.youtube.com
Temperature and Solubility Solids and Gases YouTube Examples Of Gases Soluble In Water Bringing a liquid to its boiling point will completely remove a gaseous solute. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. At 20 °c, the concentration of dissolved oxygen in water. Solubilities given for those gases which react with water, namely ozone,. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation ID3105812 Examples Of Gases Soluble In Water Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. At 20 °c, the concentration of dissolved oxygen in water. Solubilities given for those gases which. Examples Of Gases Soluble In Water.
From www.researchgate.net
CO2 solubility as a function of water depth (m) and temperature (°C Examples Of Gases Soluble In Water Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. The solubility of gases in liquid is affected by pressure and temperature. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. At 20 °c, the concentration of dissolved oxygen in. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Examples Of Gases Soluble In Water Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3. Examples Of Gases Soluble In Water.
From slideplayer.com
8.5 and Saturation. ppt download Examples Of Gases Soluble In Water Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. Bringing a liquid to its boiling point will completely remove a gaseous solute. Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. At 20 °c, the concentration of dissolved oxygen in water. Some typical. Examples Of Gases Soluble In Water.
From www.slideshare.net
Solutions Powerpoint Examples Of Gases Soluble In Water Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. At 20 °c, the concentration of dissolved oxygen in water. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Bringing a liquid. Examples Of Gases Soluble In Water.
From www.youtube.com
Solubility of Gases in Water (O2, N2, etc.) YouTube Examples Of Gases Soluble In Water Bringing a liquid to its boiling point will completely remove a gaseous solute. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. The solubility of gases in liquid is affected by pressure and temperature. At 20 °c, the concentration of dissolved oxygen in water. Solubilities given for those gases which react. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID1820539 Examples Of Gases Soluble In Water Bringing a liquid to its boiling point will completely remove a gaseous solute. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1.. Examples Of Gases Soluble In Water.
From solutionpharmacy.in
Solubility of Gas in Liquids Solution Parmacy Examples Of Gases Soluble In Water Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. Understanding the water solubility of gases in produced water is critical for piping and equipment sizing to ensure there is adequate vapor handling capacity, for example,. Solubilities given. Examples Of Gases Soluble In Water.
From www.reddit.com
Solubility of Gases in Water r/Phizze Examples Of Gases Soluble In Water The solubility of gases in liquid is affected by pressure and temperature. Bringing a liquid to its boiling point will completely remove a gaseous solute. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. At 20 °c, the concentration of dissolved oxygen in water. Solubilities given for those gases which react. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID5581895 Examples Of Gases Soluble In Water Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. The solubility of gases in liquid is affected by pressure and temperature. At 20 °c, the concentration of dissolved oxygen in water. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 ×. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Examples Of Gases Soluble In Water Bringing a liquid to its boiling point will completely remove a gaseous solute. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1.. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT CTC 450 Review PowerPoint Presentation ID4256910 Examples Of Gases Soluble In Water Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID5852920 Examples Of Gases Soluble In Water Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. At 20 °c, the concentration of dissolved oxygen in water. The solubility of gases in liquid is affected by pressure and. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Examples Of Gases Soluble In Water At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and. Examples Of Gases Soluble In Water.
From www.youtube.com
6 Gas Solubility YouTube Examples Of Gases Soluble In Water Bringing a liquid to its boiling point will completely remove a gaseous solute. Examples of gases that dissolve in water are carbon dioxide, ammonia, chlorine, and oxygen. The solubility of gases in liquid is affected by pressure and temperature. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will. Solubilities given for. Examples Of Gases Soluble In Water.
From www.slideserve.com
PPT Gases in Solution PowerPoint Presentation, free download ID5701906 Examples Of Gases Soluble In Water Solubilities given for those gases which react with water, namely ozone, nitrogen oxides, chlorine and its oxides, carbon dioxide, hydrogen sulfide,. At 20 °c, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kpa is 1.38 × 10 −3 mol l −1. The solubility of gases in liquid is affected by pressure. Examples Of Gases Soluble In Water.