Copper Hydroxide And Nitric Acid . Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. It is then converted to copper (ii). Enter an equation of an ionic chemical equation and press the balance button. Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. The balanced equation will be calculated along with the. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. In this reaction, copper is oxidized to its +2 oxidation. Copper metal dissolves in nitric acid (hno 3). In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. However, it does react with nitric acid. The two ions combine together to form an insoluble salt.
from www.youtube.com
Copper metal dissolves in nitric acid (hno 3). The two ions combine together to form an insoluble salt. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. In this reaction, copper is oxidized to its +2 oxidation. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. However, it does react with nitric acid.
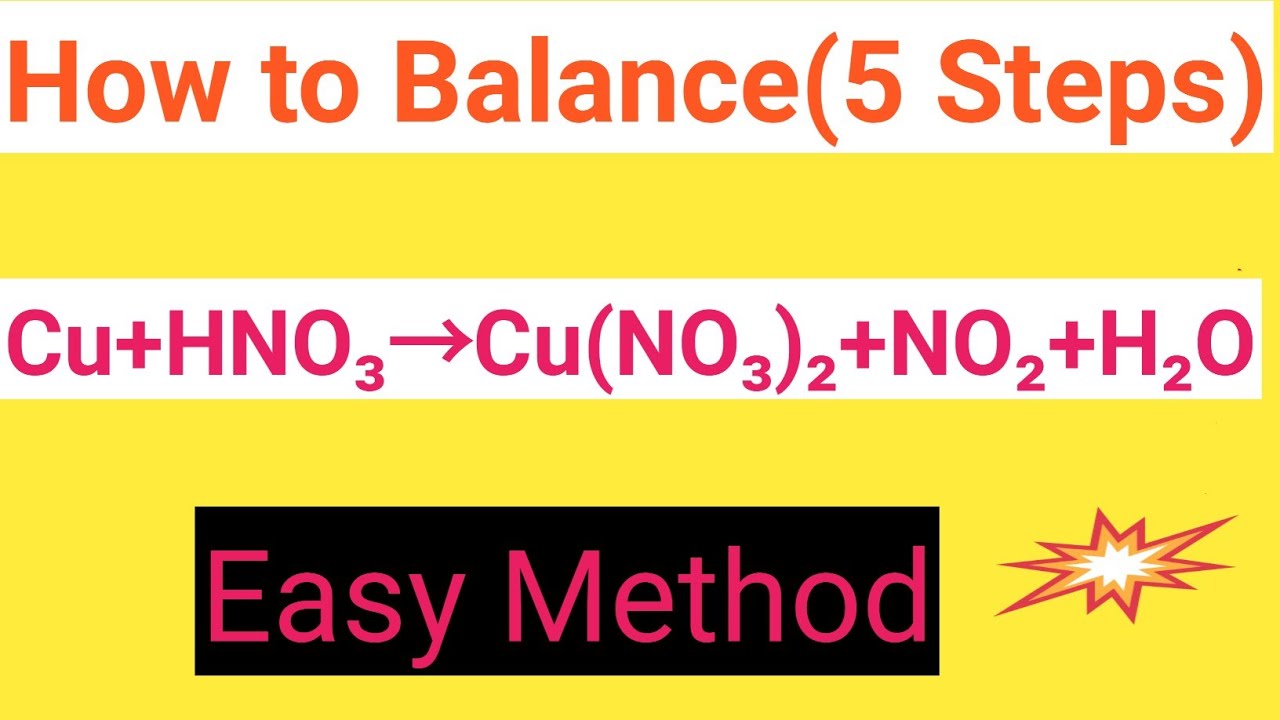
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper
Copper Hydroxide And Nitric Acid Copper metal dissolves in nitric acid (hno 3). Copper metal dissolves in nitric acid (hno 3). However, it does react with nitric acid. Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: Enter an equation of an ionic chemical equation and press the balance button. Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. It is then converted to copper (ii). The two ions combine together to form an insoluble salt. The balanced equation will be calculated along with the. In this reaction, copper is oxidized to its +2 oxidation.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Hydroxide And Nitric Acid It is then converted to copper (ii). Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. However, it does react with nitric acid. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. Cu (oh)2 + 2hno3 → cu. Copper Hydroxide And Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper Hydroxide And Nitric Acid In this reaction, copper is oxidized to its +2 oxidation. The two ions combine together to form an insoluble salt. Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. In the first reaction, copper metal is oxidized. Copper Hydroxide And Nitric Acid.
From www.numerade.com
SOLVED Potassium iodide and copper (II) sulfate Molecular and net Copper Hydroxide And Nitric Acid Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. Enter an equation of an ionic chemical equation and press the balance button. The two ions combine together to form an insoluble salt. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. It is then converted. Copper Hydroxide And Nitric Acid.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Hydroxide And Nitric Acid However, it does react with nitric acid. Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. Copper metal dissolves in nitric acid. Copper Hydroxide And Nitric Acid.
From brainly.in
complete the following reactions 1)reaction between nitric acid and Copper Hydroxide And Nitric Acid It is then converted to copper (ii). The balanced equation will be calculated along with the. Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: In this reaction, copper. Copper Hydroxide And Nitric Acid.
From www.numerade.com
SOLVED 1. Write the balanced equations for the following word Copper Hydroxide And Nitric Acid Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. It is then converted to copper (ii). The two ions combine together to form an insoluble salt. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. In. Copper Hydroxide And Nitric Acid.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Hydroxide And Nitric Acid In this reaction, copper is oxidized to its +2 oxidation. Copper metal dissolves in nitric acid (hno 3). 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: The balanced equation will be calculated along with the. It is then converted to copper (ii). The two ions combine together to form an insoluble salt. Copper. Copper Hydroxide And Nitric Acid.
From www.youtube.com
Copper and Nitric acid reaction YouTube Copper Hydroxide And Nitric Acid The balanced equation will be calculated along with the. Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. Copper metal dissolves in nitric acid (hno 3). Enter an equation of an ionic chemical equation and press the balance button. In the first reaction, copper. Copper Hydroxide And Nitric Acid.
From sciencenotes.org
Copper and Nitric Acid Chemistry Demonstration Copper Hydroxide And Nitric Acid Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. The balanced equation will be calculated along with the. Copper metal. Copper Hydroxide And Nitric Acid.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Hydroxide And Nitric Acid It is then converted to copper (ii). The balanced equation will be calculated along with the. However, it does react with nitric acid. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. The two ions combine together to form an insoluble salt. In this reaction, copper is oxidized to its +2 oxidation.. Copper Hydroxide And Nitric Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide And Nitric Acid It is then converted to copper (ii). In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. Copper metal dissolves in nitric acid (hno 3). The balanced equation will be calculated along with the. In this reaction, copper is oxidized to its +2 oxidation. Concentrated nitric acid reacts with copper and produce copper. Copper Hydroxide And Nitric Acid.
From www.numerade.com
SOLVED 2 REACTION I Dissolution of copper metal with nitric acid Copper Hydroxide And Nitric Acid Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. It is then converted to copper (ii). The balanced equation will be calculated along with the. Enter an equation of an ionic chemical. Copper Hydroxide And Nitric Acid.
From www.numerade.com
SOLVED Par Reaction of nitric acid and sodium hydroxide Reaction of Copper Hydroxide And Nitric Acid It is then converted to copper (ii). In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. In this reaction, copper is oxidized to its +2 oxidation. Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. The two ions. Copper Hydroxide And Nitric Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide And Nitric Acid 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. In this reaction, copper is oxidized to its +2 oxidation. The balanced equation will be calculated along with the. Copper metal dissolves in nitric acid (hno 3). Cu. Copper Hydroxide And Nitric Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide And Nitric Acid The two ions combine together to form an insoluble salt. Enter an equation of an ionic chemical equation and press the balance button. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. Copper metal dissolves in nitric acid (hno 3). However, it does react with nitric acid. 1 write the balanced chemical. Copper Hydroxide And Nitric Acid.
From sciencenotes.org
Copper and Nitric Acid Chemistry Demonstration Copper Hydroxide And Nitric Acid Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. Copper metal dissolves in nitric acid (hno 3). The balanced equation will be calculated along with the. However, it does react with nitric acid. It is then converted to copper (ii). Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3). Copper Hydroxide And Nitric Acid.
From www.youtube.com
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper Copper Hydroxide And Nitric Acid Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. The two ions combine together to form an insoluble salt. Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Copper metal dissolves in nitric. Copper Hydroxide And Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper Hydroxide And Nitric Acid Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. In this reaction, copper is oxidized to its +2 oxidation. Actually, the. Copper Hydroxide And Nitric Acid.
From www.youtube.com
Efficiently Recover Nitric Acid and Copper Metal From Copper Nitrate Copper Hydroxide And Nitric Acid However, it does react with nitric acid. In this reaction, copper is oxidized to its +2 oxidation. Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. In the first reaction, copper metal is oxidized by nitric acid to. Copper Hydroxide And Nitric Acid.
From brainly.in
Balance the equation Copper Hydroxide + nitric acid …… Copper nitrate Copper Hydroxide And Nitric Acid Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. The balanced equation will be calculated along with the. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. Enter an equation of an ionic. Copper Hydroxide And Nitric Acid.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Hydroxide And Nitric Acid Enter an equation of an ionic chemical equation and press the balance button. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o.. Copper Hydroxide And Nitric Acid.
From www.sciencephoto.com
Copper Reacts With Nitric Acid Stock Image C001/7513 Science Copper Hydroxide And Nitric Acid Copper metal dissolves in nitric acid (hno 3). In this reaction, copper is oxidized to its +2 oxidation. The balanced equation will be calculated along with the. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. Enter an equation of an ionic chemical equation and. Copper Hydroxide And Nitric Acid.
From www.youtube.com
Measuring the copper content of Brass (Dissolving copper with nitric Copper Hydroxide And Nitric Acid Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. The two ions combine together to form an insoluble salt. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: It is then converted to copper (ii). However, it does react with nitric acid. Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no. Copper Hydroxide And Nitric Acid.
From www.alamy.com
Concentrated Nitric Acid Reacting with Copper and Generating Nitrogen Copper Hydroxide And Nitric Acid It is then converted to copper (ii). Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. The two ions combine together to form an insoluble salt. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. Copper metal dissolves in nitric acid (hno 3). Actually, the nitrate ion oxidizes the copper metal to. Copper Hydroxide And Nitric Acid.
From www.sciencephoto.com
Copper reacts with nitric acid, 1 of 3 Stock Image C044/5795 Copper Hydroxide And Nitric Acid Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. The balanced equation will be calculated along with the. However, it does react with nitric acid. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. The two ions combine together to form an insoluble salt. Concentrated nitric acid. Copper Hydroxide And Nitric Acid.
From www.numerade.com
SOLVED After the dissolution of the copper in nitric acid, sodium Copper Hydroxide And Nitric Acid However, it does react with nitric acid. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: Copper metal dissolves in nitric acid (hno 3). Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. In this reaction, copper is oxidized to its +2 oxidation. In the first reaction, copper metal is oxidized by nitric acid. Copper Hydroxide And Nitric Acid.
From www.sciencephoto.com
Copper reacting with nitric acid Stock Image C023/0596 Science Copper Hydroxide And Nitric Acid The two ions combine together to form an insoluble salt. Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. Copper metal dissolves in nitric acid (hno 3). It is then converted to copper (ii). However, it does react with nitric acid. 1 write the balanced chemical. Copper Hydroxide And Nitric Acid.
From www.youtube.com
06 Copper nitrate and Sodium hydroxide YouTube Copper Hydroxide And Nitric Acid In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. In this reaction, copper is oxidized to its +2 oxidation. The balanced equation will be calculated along with the. However, it does react with nitric acid. Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no. Copper Hydroxide And Nitric Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide And Nitric Acid Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2),. Copper Hydroxide And Nitric Acid.
From byjus.com
Copper reacts with dilute nitric acid and forms copper nitrate, nitric Copper Hydroxide And Nitric Acid Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. The two ions combine together to form an insoluble salt. It is then converted to copper (ii). In this reaction, copper is oxidized to its +2 oxidation.. Copper Hydroxide And Nitric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Copper Hydroxide And Nitric Acid It is then converted to copper (ii). The two ions combine together to form an insoluble salt. Actually, the nitrate ion oxidizes the copper metal to copper (ii) ion while itself being transformed. Concentrated nitric acid reacts with copper and produce copper nitrate ( cu(no 3) 2), nitrogen dioxide (no 2) gas and water as products. The balanced equation will. Copper Hydroxide And Nitric Acid.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Hydroxide And Nitric Acid Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: The balanced equation will be calculated along with the. Copper metal dissolves in nitric acid (hno 3). The two ions combine together to form an insoluble salt. In the first reaction, copper metal is oxidized by. Copper Hydroxide And Nitric Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide And Nitric Acid Enter an equation of an ionic chemical equation and press the balance button. Copper metal dissolves in nitric acid (hno 3). Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: Concentrated nitric acid reacts with copper and produce copper nitrate. Copper Hydroxide And Nitric Acid.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Hydroxide And Nitric Acid Copper metal dissolves in nitric acid (hno 3). Cu (oh)2 + 2hno3 → cu (no3)2 + 2h2o. In this reaction, copper is oxidized to its +2 oxidation. The two ions combine together to form an insoluble salt. It is then converted to copper (ii). Copper is an unreactive metal and doesn’t react in normal circumstances with dilute acids. Concentrated nitric. Copper Hydroxide And Nitric Acid.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Hydroxide And Nitric Acid 1 write the balanced chemical equation for the reaction between copper hydroxide and nitric acid: However, it does react with nitric acid. Copper metal dissolves in nitric acid (hno 3). In the first reaction, copper metal is oxidized by nitric acid to form copper (ii) nitrate, cu(no3)2. It is then converted to copper (ii). Enter an equation of an ionic. Copper Hydroxide And Nitric Acid.