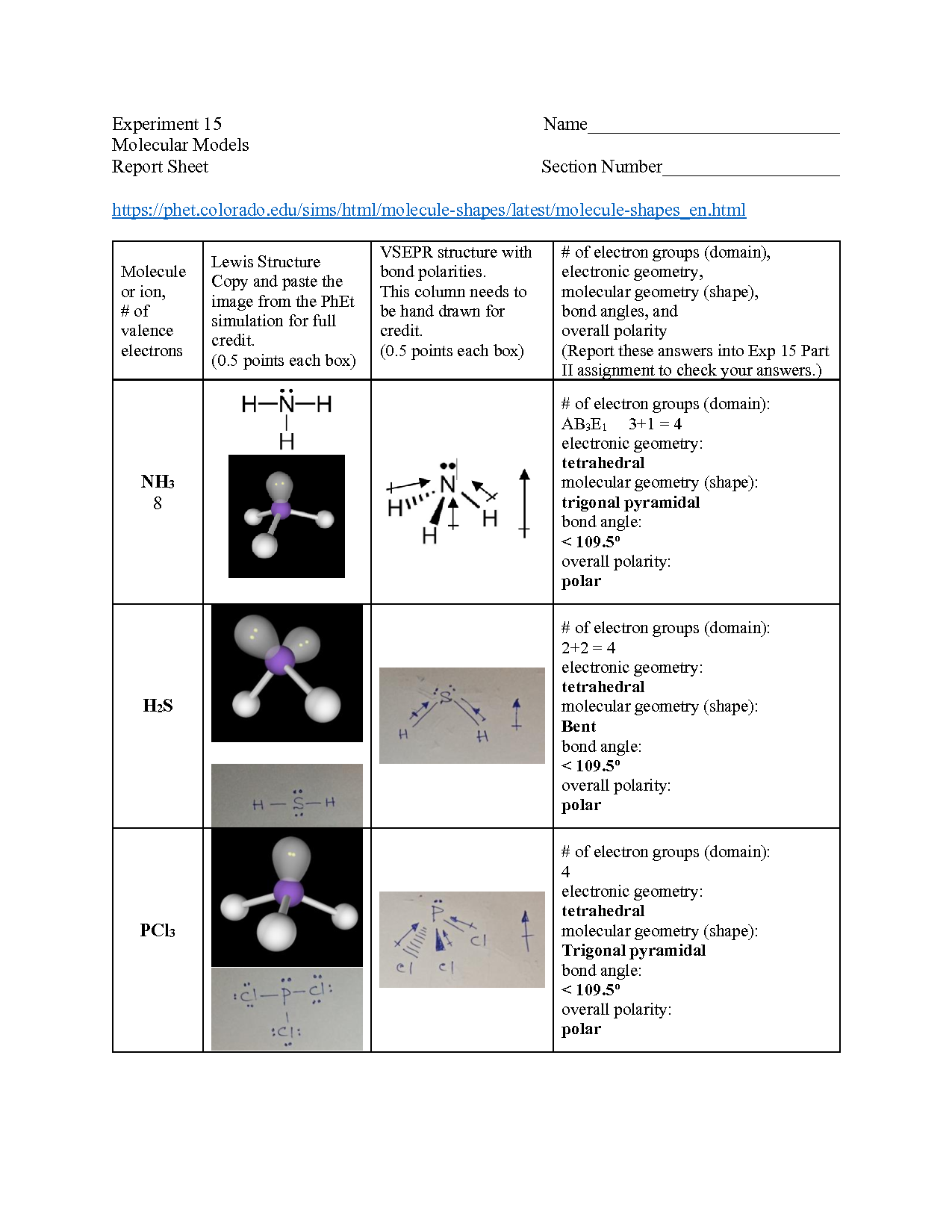
Vsepr Molecular Models Lab Answer Key . Explore molecule shapes by building molecules in 3d! Classify the vsepr model of a molecule by matching it to the following: In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Find out by adding single, double or triple bonds. Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. How does molecule shape change with different numbers of bonds and electron pairs? Determine whether bond angles are ideal (90o, 109, 120o, 180o). Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions.
from worksheets.clipart-library.com
Classify the vsepr model of a molecule by matching it to the following: Find out by adding single, double or triple bonds. Determine whether bond angles are ideal (90o, 109, 120o, 180o). In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. How does molecule shape change with different numbers of bonds and electron pairs? Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Explore molecule shapes by building molecules in 3d!
VSEPR Theory Chart & Model Lesson Worksheets Library
Vsepr Molecular Models Lab Answer Key Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. Explore molecule shapes by building molecules in 3d! Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. Determine whether bond angles are ideal (90o, 109, 120o, 180o). How does molecule shape change with different numbers of bonds and electron pairs? Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Find out by adding single, double or triple bonds. Classify the vsepr model of a molecule by matching it to the following:
From www.studypool.com
SOLUTION CHEM 10 SMC Lewis Structures and Molecular Shapes Worksheet Vsepr Molecular Models Lab Answer Key Classify the vsepr model of a molecule by matching it to the following: How does molecule shape change with different numbers of bonds and electron pairs? Explore molecule shapes by building molecules in 3d! Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. In this lab exercise you will draw lewis dot structures of a wide variety of molecules. Vsepr Molecular Models Lab Answer Key.
From mungfali.com
Molecular Geometry Worksheet Answer Key, Vsepr Wkst 1 A88 Vsepr Molecular Models Lab Answer Key Classify the vsepr model of a molecule by matching it to the following: Determine whether bond angles are ideal (90o, 109, 120o, 180o). Find out by adding single, double or triple bonds. Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules. Vsepr Molecular Models Lab Answer Key.
From mungfali.com
Molecular Geometry Worksheet Answer Key, Vsepr Wkst 1 A88 Vsepr Molecular Models Lab Answer Key The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. How does molecule shape change with different numbers of bonds and electron pairs? In this lab exercise you will draw lewis dot structures of a wide variety. Vsepr Molecular Models Lab Answer Key.
From www.studypool.com
SOLUTION VSEPR Theory Molecular Shapes Worksheet Studypool Vsepr Molecular Models Lab Answer Key Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Explore molecule shapes by building molecules in 3d! Classify the vsepr model of a molecule by matching it to the following: How does molecule shape change with different numbers of bonds and electron pairs? The vsepr model can predict the structure of. Vsepr Molecular Models Lab Answer Key.
From chem.libretexts.org
13.13 Molecular Structure The VSEPR Model Chemistry LibreTexts Vsepr Molecular Models Lab Answer Key Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. How does molecule shape change with different numbers of bonds and electron pairs? The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Classify the vsepr. Vsepr Molecular Models Lab Answer Key.
From goodimg.co
️Molecular Models Worksheet Answers Free Download Goodimg.co Vsepr Molecular Models Lab Answer Key Classify the vsepr model of a molecule by matching it to the following: Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. Explore molecule shapes by building molecules in 3d! Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Find out by adding single, double or triple bonds. Determine. Vsepr Molecular Models Lab Answer Key.
From www.chegg.com
Solved MOLECULAR MODELING LAB. Use the key below Vsepr Molecular Models Lab Answer Key Determine whether bond angles are ideal (90o, 109, 120o, 180o). Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. Classify the vsepr model of a molecule by matching it to the following: The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the. Vsepr Molecular Models Lab Answer Key.
From www.vrogue.co
Molecular Geometry And Vsepr Theory Molecular Models vrogue.co Vsepr Molecular Models Lab Answer Key Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. Classify the vsepr model of a molecule by matching it to the following: Explore molecule shapes by building molecules in 3d! In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. Determine whether bond angles are ideal. Vsepr Molecular Models Lab Answer Key.
From www.chegg.com
Solved Lab 5 Molecular Models Concepts to explore Vsepr Molecular Models Lab Answer Key Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. Classify the vsepr model of a molecule by matching it to the following: In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. How does molecule. Vsepr Molecular Models Lab Answer Key.
From mrklotzswebpage.weebly.com
Bonding, Chemical Nomenclature, and VSEPR GT Mr. Klotz's Page Vsepr Molecular Models Lab Answer Key Explore molecule shapes by building molecules in 3d! Classify the vsepr model of a molecule by matching it to the following: In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. How does molecule shape change with different numbers of bonds and electron pairs? Vsepr and molecular. Vsepr Molecular Models Lab Answer Key.
From answerfullallan101.z19.web.core.windows.net
Molecular Shapes Simulation Worksheet Answers Vsepr Molecular Models Lab Answer Key Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. Determine whether bond angles are ideal (90o, 109, 120o, 180o). Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Find out by adding single, double or triple bonds. How does. Vsepr Molecular Models Lab Answer Key.
From answerfullcopeland.z21.web.core.windows.net
Vsepr Molecular Models Lab Answer Key Vsepr Molecular Models Lab Answer Key Determine whether bond angles are ideal (90o, 109, 120o, 180o). In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. How does molecule shape change with different numbers of bonds and electron pairs? Classify the vsepr model of a molecule by matching it to the following: Now. Vsepr Molecular Models Lab Answer Key.
From www.chegg.com
Solved VSEPR Molecular Models Lab Objective To predict Vsepr Molecular Models Lab Answer Key Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. Classify the vsepr model of a molecule by matching it to the following: Vsepr and molecular modeling, distance learning. Vsepr Molecular Models Lab Answer Key.
From studylib.net
vsepr phet lab Vsepr Molecular Models Lab Answer Key Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. Explore molecule shapes by building molecules in 3d! Find out by adding single, double or triple bonds. The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic. Vsepr Molecular Models Lab Answer Key.
From davida.davivienda.com
Molecular Polarity Worksheet Answer Key Printable Word Searches Vsepr Molecular Models Lab Answer Key Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. Determine whether bond angles are ideal (90o, 109, 120o, 180o). Vsepr and molecular modeling, distance learning fall 2020, accessed. Vsepr Molecular Models Lab Answer Key.
From learningauttranadhie1h.z21.web.core.windows.net
Molecular Geometry Worksheets Vsepr Molecular Models Lab Answer Key The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. Determine whether bond angles are ideal (90o, 109, 120o, 180o). How does molecule shape change with different. Vsepr Molecular Models Lab Answer Key.
From gbu-taganskij.ru
SOLVED Molecular Geometries Lab Report Sheet Formula Lewis, 60 OFF Vsepr Molecular Models Lab Answer Key Determine whether bond angles are ideal (90o, 109, 120o, 180o). Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Explore molecule shapes by building molecules in 3d! How does molecule shape change with different numbers of bonds and electron pairs? Classify. Vsepr Molecular Models Lab Answer Key.
From www.housview.com
Vsepr Worksheets With Answers Vsepr Molecular Models Lab Answer Key Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Classify the vsepr model of a molecule by matching it to the following: Now that we. Vsepr Molecular Models Lab Answer Key.
From myans.bhantedhammika.net
Shapes Of Molecules Lab Answers Vsepr Molecular Models Lab Answer Key Classify the vsepr model of a molecule by matching it to the following: The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Determine whether bond angles are ideal (90o, 109, 120o, 180o). Linear, bent, trigonal planar,. Vsepr Molecular Models Lab Answer Key.
From mungfali.com
Molecular Geometry Worksheet Answer Key, Vsepr Wkst 1 A88 Vsepr Molecular Models Lab Answer Key Classify the vsepr model of a molecule by matching it to the following: Explore molecule shapes by building molecules in 3d! Find out by adding single, double or triple bonds. Determine whether bond angles are ideal (90o, 109, 120o, 180o). Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. How does molecule shape change with different numbers of bonds. Vsepr Molecular Models Lab Answer Key.
From www.chegg.com
Solved ime CHEM&121 Lab 4 VSEPR Models & Polarity of Vsepr Molecular Models Lab Answer Key The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Find out by adding single, double or triple bonds. In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build. Vsepr Molecular Models Lab Answer Key.
From www.shapesworksheets.com
Solved VSEPR Lab GUIDE Name Crude Model Sketch Electron Dot Chegg Vsepr Molecular Models Lab Answer Key Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds. Classify the vsepr model of a molecule by matching it to the following: In this lab exercise you will draw. Vsepr Molecular Models Lab Answer Key.
From www.studocu.com
Matias psy193 Laboratory 4 molecular geometry Name Section Date Vsepr Molecular Models Lab Answer Key Explore molecule shapes by building molecules in 3d! Find out by adding single, double or triple bonds. How does molecule shape change with different numbers of bonds and electron pairs? In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. Now that we have an understanding of. Vsepr Molecular Models Lab Answer Key.
From materialliboster.z19.web.core.windows.net
Molecular Geometry Worksheet Answer Key Vsepr Molecular Models Lab Answer Key The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Determine whether bond angles are ideal (90o, 109, 120o, 180o). Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules. Vsepr Molecular Models Lab Answer Key.
From www.studocu.com
Vsepr Lab Activity VSEPR Geometry Using Models Gather together the Vsepr Molecular Models Lab Answer Key Find out by adding single, double or triple bonds. Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. Classify the vsepr model of a molecule by matching it to the following: Explore molecule shapes by building molecules in 3d! Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. In this lab exercise you will draw lewis dot. Vsepr Molecular Models Lab Answer Key.
From www.scribd.com
VSEPR Lab Activity ANSWER KEY PDF Chemical Bond Molecules Vsepr Molecular Models Lab Answer Key Explore molecule shapes by building molecules in 3d! In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. How does molecule shape change with different numbers of bonds and electron pairs? Determine whether bond angles are ideal. Vsepr Molecular Models Lab Answer Key.
From worksheets.clipart-library.com
VSEPR Theory Chart & Model Lesson Worksheets Library Vsepr Molecular Models Lab Answer Key How does molecule shape change with different numbers of bonds and electron pairs? Determine whether bond angles are ideal (90o, 109, 120o, 180o). Explore molecule shapes by building molecules in 3d! Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. The. Vsepr Molecular Models Lab Answer Key.
From www.docsity.com
Molecular Geometry Worksheet Answer Key Exercises Chemistry Docsity Vsepr Molecular Models Lab Answer Key Classify the vsepr model of a molecule by matching it to the following: How does molecule shape change with different numbers of bonds and electron pairs? Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Find out by adding single, double or triple bonds. Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral,. Vsepr Molecular Models Lab Answer Key.
From classmediafisher.z13.web.core.windows.net
Vsepr Is Based Upon What Principle Vsepr Molecular Models Lab Answer Key Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. Classify the vsepr model of a molecule by matching it to the following: Explore molecule shapes by building molecules in 3d! Determine whether bond angles are ideal (90o, 109, 120o, 180o). Find out by adding single, double or triple bonds. Vsepr and molecular modeling, distance learning fall 2020, accessed via. Vsepr Molecular Models Lab Answer Key.
From www.studocu.com
Chem 1411 Molecular Models Lab Molecular Models Lab Report Sheet Part Vsepr Molecular Models Lab Answer Key The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Explore molecule shapes by building molecules in 3d! Classify the vsepr model of a molecule by matching it to the following: Find out by adding single, double. Vsepr Molecular Models Lab Answer Key.
From mariaafsimkins.blob.core.windows.net
Building Molecular Models Lab Answers at mariaafsimkins blog Vsepr Molecular Models Lab Answer Key How does molecule shape change with different numbers of bonds and electron pairs? Determine whether bond angles are ideal (90o, 109, 120o, 180o). Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, bipyramidal, or octahedral. In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to. Find out by adding. Vsepr Molecular Models Lab Answer Key.
From www.chegg.com
Solved VSEPR Molecular Models Lab Objective To predict Vsepr Molecular Models Lab Answer Key Find out by adding single, double or triple bonds. Determine whether bond angles are ideal (90o, 109, 120o, 180o). Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. In this lab exercise you will draw lewis dot structures of a wide variety of molecules and build three dimensional molecular models to.. Vsepr Molecular Models Lab Answer Key.
From www.vrogue.co
Vsepr Theory And Molecular Geometry Worksheet And Che vrogue.co Vsepr Molecular Models Lab Answer Key Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. Determine whether bond angles are ideal (90o, 109, 120o,. Vsepr Molecular Models Lab Answer Key.
From www.transtutors.com
(Solved) REPORT SHEET Molecular Geometries Of Covalent Molecules Vsepr Molecular Models Lab Answer Key Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Explore molecule shapes by building molecules in 3d! The vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions. In. Vsepr Molecular Models Lab Answer Key.
From www.chegg.com
Solved CHEM 121 Lab Moal Revised 05 2017 Chemical Bonding Vsepr Molecular Models Lab Answer Key Classify the vsepr model of a molecule by matching it to the following: How does molecule shape change with different numbers of bonds and electron pairs? Vsepr and molecular modeling, distance learning fall 2020, accessed via blackboard. Determine whether bond angles are ideal (90o, 109, 120o, 180o). Find out by adding single, double or triple bonds. Linear, bent, trigonal planar,. Vsepr Molecular Models Lab Answer Key.