When Does Water Boil Over . The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. When cold water is poured over the top of the flask, it cools the gas above the liquid water. Is it more likely to happen with certain ingredients?. How can you stop it from happening? What are the mechanics of water boiling over?
from es.hinative.com
The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. How can you stop it from happening? Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. Is it more likely to happen with certain ingredients?. What are the mechanics of water boiling over? When cold water is poured over the top of the flask, it cools the gas above the liquid water.
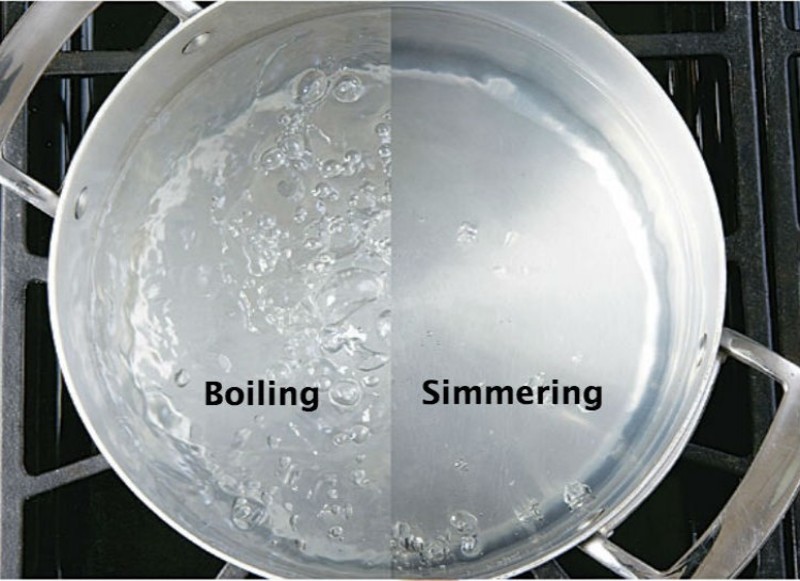
¿Cuál es la diferencia entre "simmer" y "boil" ? "simmer" vs "boil
When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. When cold water is poured over the top of the flask, it cools the gas above the liquid water. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. How can you stop it from happening? What are the mechanics of water boiling over? The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. Is it more likely to happen with certain ingredients?.
From cookthink.com
How Long Does it Take for Water to Boil CookThink When Does Water Boil Over Is it more likely to happen with certain ingredients?. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. How can you stop it from happening? From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. Water boils at. When Does Water Boil Over.
From www.waterev.com
How to stop water from boiling over When Does Water Boil Over Is it more likely to happen with certain ingredients?. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. Water boils at a lower temperature as you gain. When Does Water Boil Over.
From ar.inspiredpencil.com
Boiling Point Of Water Fahrenheit When Does Water Boil Over When cold water is poured over the top of the flask, it cools the gas above the liquid water. The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. How can. When Does Water Boil Over.
From www.epicurious.com
Why the Meyer Stockpot Helps Prevent BoilOvers Epicurious When Does Water Boil Over The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s. When Does Water Boil Over.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly When Does Water Boil Over Is it more likely to happen with certain ingredients?. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. How can you stop it from happening?. When Does Water Boil Over.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation When Does Water Boil Over Is it more likely to happen with certain ingredients?. How can you stop it from happening? The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. What are the mechanics of water boiling over? The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure. When Does Water Boil Over.
From www.yiannislucacos.gr
How to boil Boiling as a basic cooking method Yiannis Lucacos When Does Water Boil Over When cold water is poured over the top of the flask, it cools the gas above the liquid water. Is it more likely to happen with certain ingredients?. The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. The boiling point of a liquid depends on temperature, atmospheric pressure,. When Does Water Boil Over.
From es.hinative.com
¿Cuál es la diferencia entre "simmer" y "boil" ? "simmer" vs "boil When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. In the video, water is boiled in a flask, which is then stoppered and. When Does Water Boil Over.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly When Does Water Boil Over The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. How can you stop it from happening? When cold water is poured over the top of the flask, it cools the gas above the liquid water. The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard. When Does Water Boil Over.
From www.youtube.com
Why does water boil in a vacuum? YouTube When Does Water Boil Over From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. In the video, water is boiled in a flask, which is then. When Does Water Boil Over.
From thermtest.com
Does Adding Salt to Water Help it Boil Faster? and Why? When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. When cold water is poured over the top of the flask, it cools the gas above the liquid water. Water boils. When Does Water Boil Over.
From www.cuisinecravings.com
Does Water Boil Faster With a Lid? How to Boil Water Faster? When Does Water Boil Over Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor. When Does Water Boil Over.
From caseoftheyums.com
Why isn’t Your Water Boiling? Case of The Yums When Does Water Boil Over From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. In the video, water is boiled in a flask, which is then stoppered and removed from. When Does Water Boil Over.
From chefd.com
What Temperature Does Water Boil? Chefd When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary. When Does Water Boil Over.
From www.foodrepublic.com
The Brilliant Butter Hack To Prevent Water From Boiling Over When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. When cold water is poured over the top of the flask, it cools the gas above the liquid water. How can. When Does Water Boil Over.
From sciencenotes.org
Does Boiling Water Keep Getting Hotter? When Does Water Boil Over What are the mechanics of water boiling over? Is it more likely to happen with certain ingredients?. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. When cold water is poured over the top of the flask, it cools the gas above the liquid water. How. When Does Water Boil Over.
From indianapublicmedia.org
Cooking Question When Is Water Actually Boiling? A Moment of Science When Does Water Boil Over What are the mechanics of water boiling over? In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. How can you stop it from happening? When cold water is poured over the top of the flask, it cools the gas above the liquid water. The boiling point of a liquid depends. When Does Water Boil Over.
From sciencenotes.org
How to Boil Water at Room Temperature When Does Water Boil Over The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. What are the mechanics of water boiling over? Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. In the video, water is boiled in a flask,. When Does Water Boil Over.
From www.allrecipes.com
How to Boil Water Faster Allrecipes When Does Water Boil Over How can you stop it from happening? In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. The boiling point of a liquid depends on temperature, atmospheric pressure, and the. When Does Water Boil Over.
From crescentcity-fl.com
Boil Water Notices Crescent City, Florida When Does Water Boil Over How can you stop it from happening? In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. When cold water is poured over the top of. When Does Water Boil Over.
From purewaterblog.com
Does Boiling Water Remove Chlorine from Drinking Water? Water Treatment When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. What are the mechanics of water boiling over? How can you stop it from happening? Is it more likely to happen with certain ingredients?. From the highest land point above sea level, mount everest, to the lowest, the dead. When Does Water Boil Over.
From thecookwaregeek.com
How Long Does it Take to Boil Water? The Cookware Geek When Does Water Boil Over Is it more likely to happen with certain ingredients?. What are the mechanics of water boiling over? Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. In the video, water is boiled in a flask, which is then stoppered and removed from the heat. When Does Water Boil Over.
From www.mentalfloss.com
Does Adding Salt to Water Make It Boil Sooner? Mental Floss When Does Water Boil Over What are the mechanics of water boiling over? Is it more likely to happen with certain ingredients?. How can you stop it from happening? Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. When cold water is poured over the top of the flask,. When Does Water Boil Over.
From ddojahyneco.blob.core.windows.net
What Does It Look Like When Water Is Boiled at Ruth Marquis blog When Does Water Boil Over Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. Is it more likely to happen with certain ingredients?. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. The normal boiling point is the temperature. When Does Water Boil Over.
From www.seriouseats.com
Everything You Ever Wanted to Know (Plus More!) About Boiling Water When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. Is it more likely to happen with certain ingredients?. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. What are the mechanics of water boiling over? Water boils at. When Does Water Boil Over.
From www.elliotlaketoday.com
Boil water advisory lifted in Elliot Lake Elliot Lake News When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. What are the mechanics of water boiling over? Is it more likely to happen with certain ingredients?. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from. When Does Water Boil Over.
From www.livingwhole.com.au
Does boiling water remove fluoride? The Answer is No Living Whole When Does Water Boil Over In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. When cold water is poured over the top of the flask, it cools the gas above the liquid water. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from. When Does Water Boil Over.
From vandewaterbooks.com
What Temperature Does Water Boil At? Boiling Point & Elevation Vande When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. How can you stop it from happening? Is it more likely to happen with certain ingredients?. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just.. When Does Water Boil Over.
From preparedcooks.com
Does Water Boil Faster With a Lid? (Updated for 2024) When Does Water Boil Over What are the mechanics of water boiling over? How can you stop it from happening? Is it more likely to happen with certain ingredients?. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s. When Does Water Boil Over.
From foodandfizz.com
Does Water Boil Faster With The Lid On? The 1 Best Revelation When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. What are the mechanics of water boiling over? The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. Water boils at a lower temperature as you gain altitude (e.g., going higher. When Does Water Boil Over.
From aweseas.blogspot.com
Boiling Point Of Water Sea Level When Does Water Boil Over The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. How can you stop it from happening? Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. From the highest land point above sea. When Does Water Boil Over.
From foodandfizz.com
Does Water Boil Faster With A Lid? 1 Best Answer When Does Water Boil Over Is it more likely to happen with certain ingredients?. What are the mechanics of water boiling over? From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. In the video, water is boiled in a flask, which is then stoppered and removed from the heat source. How. When Does Water Boil Over.
From sciencenotes.org
What Are the Bubbles in Boiling Water? When Does Water Boil Over From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. How can you stop it from happening? When cold water is poured. When Does Water Boil Over.
From www.thenakedscientists.com
Why does pasta or rice water boil over? Science Questions When Does Water Boil Over The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. Is it more likely to happen with certain ingredients?. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. How can you stop it from happening? In. When Does Water Boil Over.
From ulesson.com
Why Does Salt Water Boil Faster? uLesson When Does Water Boil Over The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. When cold water is poured over the top of the flask, it cools the gas above the liquid water. The normal boiling point is the temperature at which the liquid’s vapour pressure equals the standard sea level atmospheric pressure (760. Is it. When Does Water Boil Over.