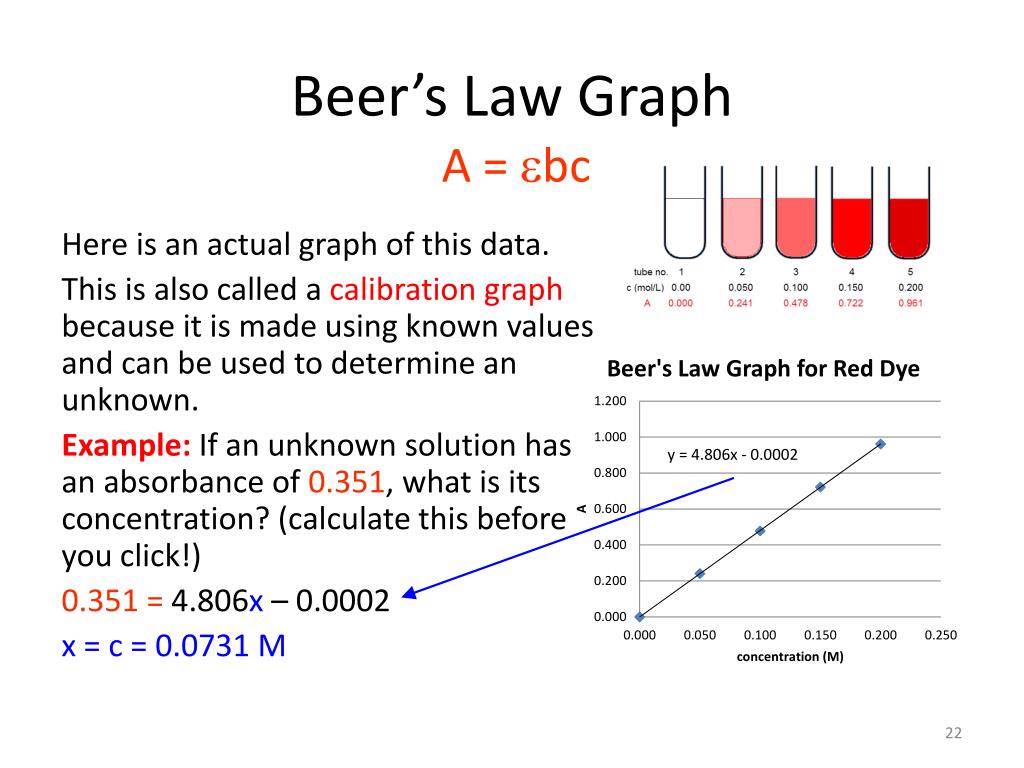
Beer Lambert Law Equation . Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A = log10(io i) =. A= εcl a = ε c l where: Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample
from
Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample “the path length and concentration of a chemical are directly proportional to its absorption of light.” A = log10(io i) =. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= εcl a = ε c l where:
Beer Lambert Law Equation “the path length and concentration of a chemical are directly proportional to its absorption of light.” A = log10(io i) =. A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A= εcl a = ε c l where: “the path length and concentration of a chemical are directly proportional to its absorption of light.”
From
Beer Lambert Law Equation A= εcl a = ε c l where: A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o. Beer Lambert Law Equation.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. Beer Lambert Law Equation.
From
Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= εcl a = ε. Beer Lambert Law Equation.
From
Beer Lambert Law Equation “the path length and concentration of a chemical are directly proportional to its absorption of light.” Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A = log10(io i) =.. Beer Lambert Law Equation.
From
Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. Beer Lambert Law Equation.
From ceajxuqm.blob.core.windows.net
Beer Lambert Law Frq at Aaron Salinas blog Beer Lambert Law Equation A = log10(io i) =. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= εcl a = ε c l where: Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c “the path length and concentration of a chemical. Beer Lambert Law Equation.
From www.slideserve.com
PPT Atmospheric Transmission PowerPoint Presentation, free download ID3975309 Beer Lambert Law Equation A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= εcl a = ε c l where: Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i. Beer Lambert Law Equation.
From
Beer Lambert Law Equation A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= εcl a = ε c l where: Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o. Beer Lambert Law Equation.
From
Beer Lambert Law Equation A= εcl a = ε c l where: “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample Beer lamberts law states a relationship between the attenuation of light through a substance and the properties. Beer Lambert Law Equation.
From
Beer Lambert Law Equation A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= εcl a = ε c l where: Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i). Beer Lambert Law Equation.
From
Beer Lambert Law Equation A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample “the path length and concentration of a chemical are directly proportional to its absorption of light.” A = log10(io i) =. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A=. Beer Lambert Law Equation.
From
Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= εcl a = ε c l where: “the path length and concentration of a chemical are directly proportional to its absorption of light.” Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a =. Beer Lambert Law Equation.
From
Beer Lambert Law Equation A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= εcl a = ε c l where: Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o. Beer Lambert Law Equation.
From
Beer Lambert Law Equation “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample A= εcl a = ε c l where: A = log10(io i) =. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o. Beer Lambert Law Equation.
From byjus.com
State and explain Beer Lambert Law. Beer Lambert Law Equation “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c Beer lamberts law states a relationship. Beer Lambert Law Equation.
From www.slideserve.com
PPT Protein Concentration Determination PowerPoint Presentation, free download ID3969127 Beer Lambert Law Equation “the path length and concentration of a chemical are directly proportional to its absorption of light.” A = log10(io i) =. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= εcl a = ε c l where: Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i. Beer Lambert Law Equation.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= εcl a = ε c l where: Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A = log10(io i) =. “the path length and concentration of a chemical. Beer Lambert Law Equation.
From dxofafnaw.blob.core.windows.net
Beer Lambert Law Definition Chemistry at Myrtle blog Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= εcl a = ε c l where: Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i. Beer Lambert Law Equation.
From
Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c “the path length and concentration of a chemical are directly proportional to its absorption of light.” A = log10(io i) =.. Beer Lambert Law Equation.
From
Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= εcl a = ε c l where: Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A = log10(io i) =. A= ϵlc a= absorbance ϵ = molar extinction. Beer Lambert Law Equation.
From www.youtube.com
Beer Lambert law in Hindi Beer Lambert Law explained Lambert beer law equation YouTube Beer Lambert Law Equation A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A = log10(io i) =. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a =. Beer Lambert Law Equation.
From www.youtube.com
Spectroscopy Beer Lambert's Law YouTube Beer Lambert Law Equation “the path length and concentration of a chemical are directly proportional to its absorption of light.” Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A= εcl a = ε. Beer Lambert Law Equation.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download ID4942160 Beer Lambert Law Equation A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.” Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A= εcl a = ε c l where: Beer lamberts law states a relationship between the attenuation of light through. Beer Lambert Law Equation.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News Beer Lambert Law Equation Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A = log10(io i) =. A= εcl a = ε c l where: “the path length and concentration of a chemical. Beer Lambert Law Equation.
From joimeihka.blob.core.windows.net
Beer Lambert Law Linear Equation at Kimberly Graves blog Beer Lambert Law Equation Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A= εcl a = ε c l where: “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of. Beer Lambert Law Equation.
From
Beer Lambert Law Equation A= εcl a = ε c l where: A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.” Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i. Beer Lambert Law Equation.
From
Beer Lambert Law Equation A= εcl a = ε c l where: A = log10(io i) =. A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical. Beer Lambert Law Equation.
From www.slideserve.com
PPT Atmospheric Transmission PowerPoint Presentation, free download ID3975309 Beer Lambert Law Equation A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample “the path length and concentration of a chemical are directly proportional to its absorption of light.” Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A = log10(io i) =.. Beer Lambert Law Equation.
From ceupbgif.blob.core.windows.net
Beer Lambert Law Light Intensity at Leisa Lemelle blog Beer Lambert Law Equation A= εcl a = ε c l where: A = log10(io i) =. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample “the path length and concentration of a chemical are. Beer Lambert Law Equation.
From
Beer Lambert Law Equation A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical are directly proportional to its absorption of light.” Log(io i) = a =. Beer Lambert Law Equation.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1) log (i o i) = a = ε l c A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample A= εcl. Beer Lambert Law Equation.
From
Beer Lambert Law Equation A = log10(io i) =. A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample A= εcl a = ε c l where: Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Log(io i) = a = εlc (11.1.1.1) (11.1.1.1). Beer Lambert Law Equation.
From
Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample A = log10(io i) =. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. Beer Lambert Law Equation.
From
Beer Lambert Law Equation Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical are directly proportional to its absorption of light.” A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample A = log10(io i) =.. Beer Lambert Law Equation.
From
Beer Lambert Law Equation “the path length and concentration of a chemical are directly proportional to its absorption of light.” A = log10(io i) =. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. A= ϵlc a= absorbance ϵ = molar extinction coefficient l = path length c = concentration of the sample. Beer Lambert Law Equation.