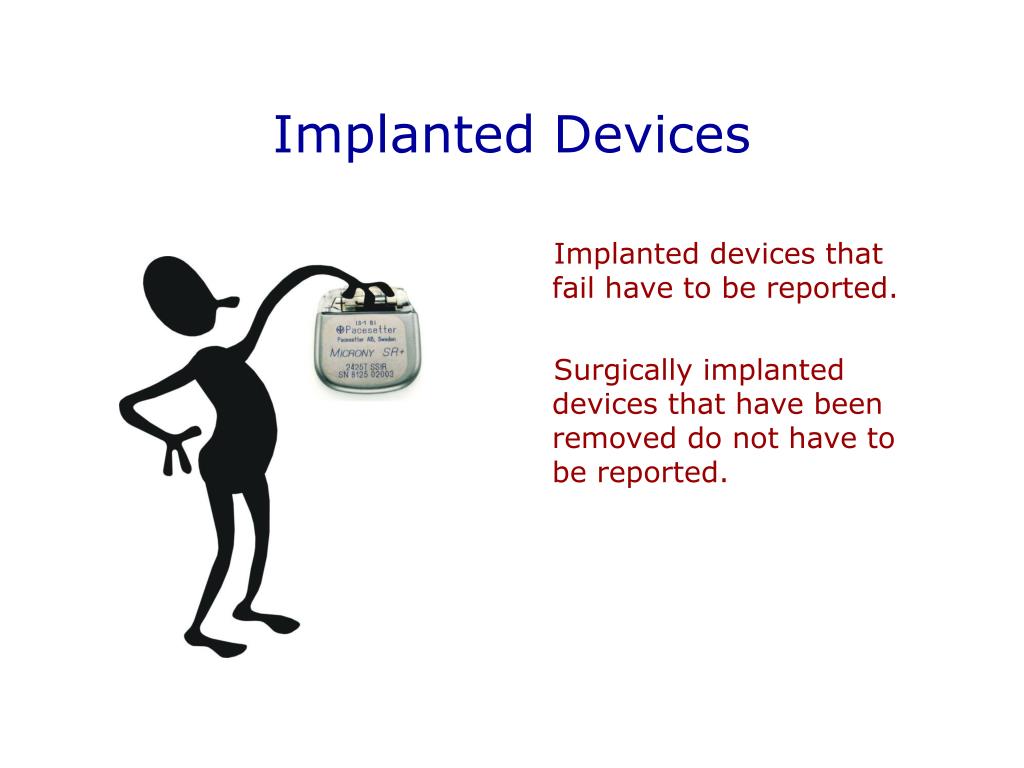
Safe Medical Device Act Devices . The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. It mandated that manufacturers and health care professionals. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,.
from www.slideserve.com
The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. It mandated that manufacturers and health care professionals. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices.
PPT Medical Equipment and the Safe Medical Device Act (SMDA
Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. It mandated that manufacturers and health care professionals.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. The safe medical devices act of 1990. Safe Medical Device Act Devices.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Safe Medical Device Act Devices The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The medical device reporting. Safe Medical Device Act Devices.
From www.slideshare.net
Safe Medical Devices Act 1990 Safe Medical Device Act Devices The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. It mandated that manufacturers and health care professionals. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. It mandated that manufacturers and health care professionals. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Safe medical device amendments of 1990 or safe medical devices act. Safe Medical Device Act Devices.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Safe Medical Device Act Devices Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. It mandated that manufacturers and health care professionals. The safe medical devices act of 1990 created important reporting requirements for the use of medical. Safe Medical Device Act Devices.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Safe Medical Device Act Devices The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Resources related. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Overview of FDA How Regulation Came to Be PowerPoint Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. It mandated that manufacturers. Safe Medical Device Act Devices.
From kladhxznh.blob.core.windows.net
Safe Medical Device Act Fda at Joshua Martin blog Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. It mandated that manufacturers and health. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. In canada, medical devices. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. It mandated that manufacturers and health care professionals. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The safe medical devices act of 1990 created important reporting requirements for the use of. Safe Medical Device Act Devices.
From kladhxznh.blob.core.windows.net
Safe Medical Device Act Fda at Joshua Martin blog Safe Medical Device Act Devices The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. It mandated that manufacturers and health care professionals. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. In. Safe Medical Device Act Devices.
From www.slideshare.net
Safe Medical Devices Act 1990 Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. In canada, medical devices. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. It mandated that manufacturers and health care professionals. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. In canada, medical devices are grouped into 4 classes based on the expected level of risk. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. It mandated that manufacturers and health care professionals. Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. Resources related to and required for sale of medical devices in canada including legislation, health. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. It mandated that manufacturers and health care professionals. The. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. It mandated that manufacturers and health care professionals. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities,. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Resources related. Safe Medical Device Act Devices.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Safe Medical Device Act Devices The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. It mandated that manufacturers and health care professionals. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. Resources related to and required for sale of medical devices in canada including legislation, health canada. Safe Medical Device Act Devices.
From kladhxznh.blob.core.windows.net
Safe Medical Device Act Fda at Joshua Martin blog Safe Medical Device Act Devices Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. It mandated that manufacturers and health care professionals. The safe medical devices act of 1990 created important reporting requirements for the use of medical. Safe Medical Device Act Devices.
From www.slideshare.net
Safe Medical Devices Act 1990 PPT Safe Medical Device Act Devices In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. Resources related to and. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. The medical device reporting. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. The medical device reporting. Safe Medical Device Act Devices.
From www.homecarepulse.com
Safe Medical Devices Act Home Care Pulse Safe Medical Device Act Devices The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. It mandated. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. It mandated that manufacturers and health care professionals. The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. The safe medical devices act of 1990 created important reporting requirements for the use of medical. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The safe medical devices. Safe Medical Device Act Devices.
From www.scribd.com
Introduction to Medical Device Act 2012 (Act 737) and Medical Device Safe Medical Device Act Devices In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. It mandated that manufacturers and health care professionals. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. The safe medical devices act of 1990 created important reporting requirements for the. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. The. Safe Medical Device Act Devices.
From www.slideshare.net
Safe Medical Devices Act 1990 Safe Medical Device Act Devices Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The safe medical devices act of. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. It mandated that manufacturers and health care professionals. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Resources related to and required for sale of medical devices in canada including legislation, health canada. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. It mandated that manufacturers and health care professionals. In. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The. Safe Medical Device Act Devices.
From www.academia.edu
(PDF) The Safe Medical Device Act Terry Donner Academia.edu Safe Medical Device Act Devices Resources related to and required for sale of medical devices in canada including legislation, health canada guidelines,. Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The safe medical devices act (smda) of 1990 provided fda with two additional postmarketing activities, postmarket surveillance for the. It mandated that manufacturers and health care professionals. The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers,. Safe Medical Device Act Devices.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Safe Medical Device Act Devices The safe medical devices act of 1990 created important reporting requirements for the use of medical devices. Safe medical device amendments of 1990 or safe medical devices act sanctioned progressive reporting and tracking rules for medical devices. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Resources. Safe Medical Device Act Devices.