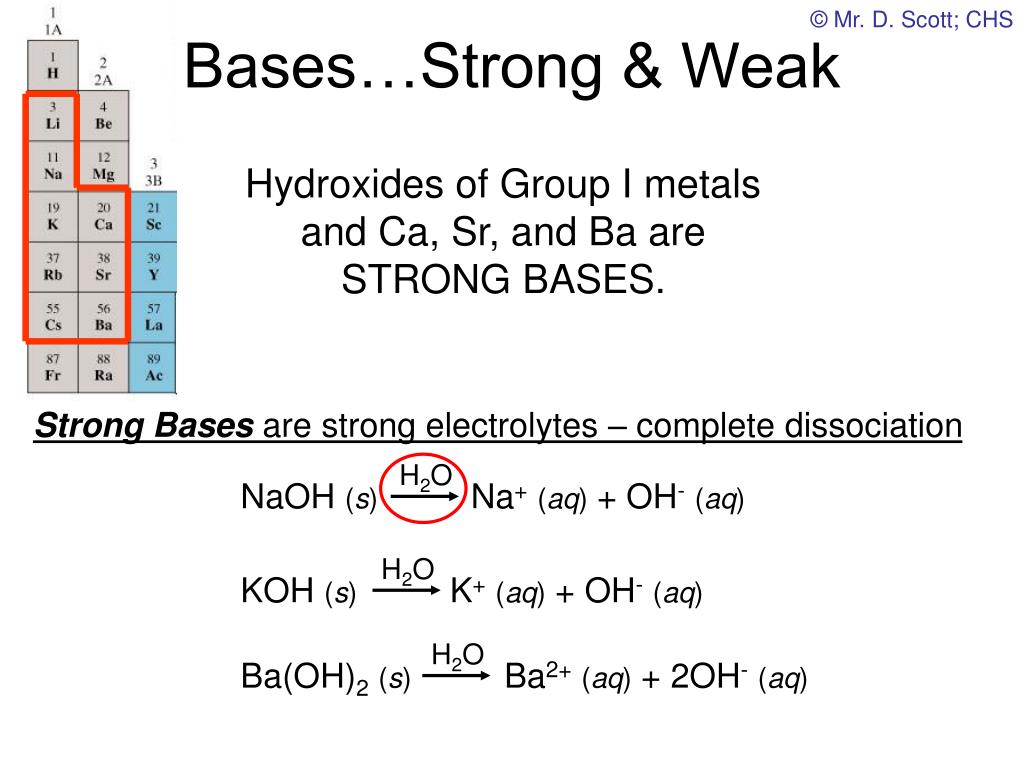
What Makes Bases Strong Or Weak . There are very few strong bases (table \(\pageindex{1}\)); As a part of this it defines and explains k b and pk b. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. We are going to use the. Any base not listed is a weak base. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. Classifying acids or bases as strong or weak has nothing to do with their. A weak acid is one that. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte is called a weak base. This property makes it a highly efficient proton acceptor and catalyst for. You can think of the compound as. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. This page explains the terms strong and weak as applied to bases.
from www.slideserve.com
A weak acid is one that. There are very few strong bases (table \(\pageindex{1}\)); This page explains the terms strong and weak as applied to bases. You can think of the compound as. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. As a part of this it defines and explains k b and pk b. Any base not listed is a weak base. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. This property makes it a highly efficient proton acceptor and catalyst for.
PPT AcidBase PowerPoint Presentation, free download ID853956
What Makes Bases Strong Or Weak As a part of this it defines and explains k b and pk b. Any base not listed is a weak base. Classifying acids or bases as strong or weak has nothing to do with their. We are going to use the. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte is called a weak base. This page explains the terms strong and weak as applied to bases. There are very few strong bases (table \(\pageindex{1}\)); As a part of this it defines and explains k b and pk b. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. A weak acid is one that. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. This property makes it a highly efficient proton acceptor and catalyst for. You can think of the compound as.
From www.youtube.com
Weak Base vs Strong BaseDifference between strong base and weak base What Makes Bases Strong Or Weak A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. You can think of the compound as. This page explains the terms strong and weak as applied to bases. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves. What Makes Bases Strong Or Weak.
From studylib.net
Strong and weak acids and bases What Makes Bases Strong Or Weak A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. Classifying acids or bases as strong or weak has nothing to do with their. We are going to use the. A strong base, unlike a weak base, dissociates (separates) completely into ions when it. What Makes Bases Strong Or Weak.
From www.youtube.com
Strong vs weak acids and bases (video) YouTube What Makes Bases Strong Or Weak A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte is called a weak base. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. This page explains the terms strong and weak as applied to bases. There are very few strong bases. What Makes Bases Strong Or Weak.
From www.thoughtco.com
List of the Strong Bases (Arrhenius Bases) What Makes Bases Strong Or Weak This page explains the terms strong and weak as applied to bases. There are very few strong bases (table \(\pageindex{1}\)); Classifying acids or bases as strong or weak has nothing to do with their. You can think of the compound as. A base that is a strong electrolyte is called a strong base, while a base that is a weak. What Makes Bases Strong Or Weak.
From www.slideserve.com
PPT Ch.9 J.C. Rowe PowerPoint Presentation, free download ID3493976 What Makes Bases Strong Or Weak This property makes it a highly efficient proton acceptor and catalyst for. A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte is called a weak base. We are going to use the. Classifying acids or bases as strong or weak has nothing to do with their. This page explains. What Makes Bases Strong Or Weak.
From www.thoughtco.com
List of Common Strong and Weak Acids What Makes Bases Strong Or Weak A weak acid is one that. This property makes it a highly efficient proton acceptor and catalyst for. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. Any base not listed is a weak base. You can think. What Makes Bases Strong Or Weak.
From pediaa.com
Difference Between Strong and Weak Bases Definition, Properties What Makes Bases Strong Or Weak You can think of the compound as. This page explains the terms strong and weak as applied to bases. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. A strong base, unlike a weak base, dissociates (separates) completely. What Makes Bases Strong Or Weak.
From www.youtube.com
Strong & Weak Acids and Bases YouTube What Makes Bases Strong Or Weak This page explains the terms strong and weak as applied to bases. A weak acid is one that. This property makes it a highly efficient proton acceptor and catalyst for. There are very few strong bases (table \(\pageindex{1}\)); A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is. What Makes Bases Strong Or Weak.
From www.chegg.com
Solved The titration of a strong acid with a weak base will What Makes Bases Strong Or Weak A weak acid is one that. This page explains the terms strong and weak as applied to bases. As a part of this it defines and explains k b and pk b. Any base not listed is a weak base. A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte. What Makes Bases Strong Or Weak.
From www.slideserve.com
PPT Acids and Bases Introduction PowerPoint Presentation, free What Makes Bases Strong Or Weak This page explains the terms strong and weak as applied to bases. A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte is called a weak base. As a part of this it defines and explains k b and pk b. A strong base, unlike a weak base, dissociates (separates). What Makes Bases Strong Or Weak.
From www.slideserve.com
PPT MODERN CHEMISTRY CHAPTER 14 ACIDS AND BASES PowerPoint What Makes Bases Strong Or Weak There are very few strong bases (table \(\pageindex{1}\)); The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. This property makes it a highly efficient proton acceptor and catalyst for. A strong base is something like sodium hydroxide or. What Makes Bases Strong Or Weak.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry What Makes Bases Strong Or Weak This page explains the terms strong and weak as applied to bases. Any base not listed is a weak base. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. There are very few strong bases (table \(\pageindex{1}\)); A weak acid is one that. Classifying acids or bases as strong or weak has. What Makes Bases Strong Or Weak.
From www.slideserve.com
PPT Ionization Constants of Acids and Bases Strengths of Acids and What Makes Bases Strong Or Weak A weak acid is one that. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas. What Makes Bases Strong Or Weak.
From www.slideserve.com
PPT Strengths of Acids and Bases PowerPoint Presentation, free What Makes Bases Strong Or Weak This page explains the terms strong and weak as applied to bases. Any base not listed is a weak base. We are going to use the. A weak acid is one that. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. This property makes it a highly efficient proton acceptor and catalyst. What Makes Bases Strong Or Weak.
From www.differencebetween.net
Difference Between Weak Base and Strong Base Difference Between What Makes Bases Strong Or Weak You can think of the compound as. Any base not listed is a weak base. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. A base that is. What Makes Bases Strong Or Weak.
From studylib.net
Strong and Weak Bases What Makes Bases Strong Or Weak Classifying acids or bases as strong or weak has nothing to do with their. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. Any base not listed is a weak base. A strong base, unlike a weak base, dissociates (separates) completely into ions. What Makes Bases Strong Or Weak.
From www.expii.com
Strong and Weak Acids and Bases — Definition & Examples Expii What Makes Bases Strong Or Weak A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. As a part of this it defines and explains k b and pk b. A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte. What Makes Bases Strong Or Weak.
From www.slideserve.com
PPT Unit 3 PowerPoint Presentation, free download ID2956486 What Makes Bases Strong Or Weak This property makes it a highly efficient proton acceptor and catalyst for. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water.. What Makes Bases Strong Or Weak.
From quizdbcornwallis.z21.web.core.windows.net
What Makes A Base Weak What Makes Bases Strong Or Weak There are very few strong bases (table \(\pageindex{1}\)); A weak acid is one that. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. This page explains the terms strong and weak as applied to bases. This property makes it a highly efficient proton acceptor and catalyst for. We are going to use the. A. What Makes Bases Strong Or Weak.
From askanydifference.com
Strong Base vs Weak Base Difference and Comparison What Makes Bases Strong Or Weak You can think of the compound as. This page explains the terms strong and weak as applied to bases. This property makes it a highly efficient proton acceptor and catalyst for. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. Classifying acids or. What Makes Bases Strong Or Weak.
From gamesmartz.com
Weak Base Easy to Understand What Makes Bases Strong Or Weak This property makes it a highly efficient proton acceptor and catalyst for. This page explains the terms strong and weak as applied to bases. As a part of this it defines and explains k b and pk b. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. A weak acid is one. What Makes Bases Strong Or Weak.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo What Makes Bases Strong Or Weak This page explains the terms strong and weak as applied to bases. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. A strong acid is one that loses h + easily, meaning that its conjugate base holds the. What Makes Bases Strong Or Weak.
From www.askdifference.com
Strong Bases vs. Weak Bases — What’s the Difference? What Makes Bases Strong Or Weak A weak acid is one that. As a part of this it defines and explains k b and pk b. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte is called a. What Makes Bases Strong Or Weak.
From kolblabs.com
Strong and Weak bases in Acids, Bases and Salts Class 10 Science What Makes Bases Strong Or Weak A weak acid is one that. As a part of this it defines and explains k b and pk b. We are going to use the. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. A base that. What Makes Bases Strong Or Weak.
From www.sliderbase.com
Buffers Presentation Chemistry What Makes Bases Strong Or Weak There are very few strong bases (table \(\pageindex{1}\)); The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. This page explains the terms strong and weak as applied to bases. You can think of the compound as. We are. What Makes Bases Strong Or Weak.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs What Makes Bases Strong Or Weak This property makes it a highly efficient proton acceptor and catalyst for. Any base not listed is a weak base. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. You can think of the compound as. A strong acid is one that loses h + easily, meaning that its conjugate base holds. What Makes Bases Strong Or Weak.
From exoczofhf.blob.core.windows.net
Weak Acid Vs Base Titration at Rico Padgett blog What Makes Bases Strong Or Weak Classifying acids or bases as strong or weak has nothing to do with their. A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. There are very few strong bases (table \(\pageindex{1}\)); This page explains the terms strong and weak as applied to bases. Any base not listed is a weak base. You. What Makes Bases Strong Or Weak.
From www.slideserve.com
PPT Chemical Reactions in Aqueous Solutions PowerPoint Presentation What Makes Bases Strong Or Weak This page explains the terms strong and weak as applied to bases. Classifying acids or bases as strong or weak has nothing to do with their. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. You can think of the compound as. A base that is a strong electrolyte is called a strong base,. What Makes Bases Strong Or Weak.
From www.slideserve.com
PPT Weak Acids Weak Bases PowerPoint Presentation, free download ID What Makes Bases Strong Or Weak A weak acid is one that. This page explains the terms strong and weak as applied to bases. This property makes it a highly efficient proton acceptor and catalyst for. You can think of the compound as. There are very few strong bases (table \(\pageindex{1}\)); A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic.. What Makes Bases Strong Or Weak.
From askanydifference.com
Strong Base vs Weak Base Difference and Comparison What Makes Bases Strong Or Weak You can think of the compound as. There are very few strong bases (table \(\pageindex{1}\)); This property makes it a highly efficient proton acceptor and catalyst for. A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte is called a weak base. A strong base, unlike a weak base, dissociates. What Makes Bases Strong Or Weak.
From www.pinterest.com
Strong and Weak Acids & Bases Chemistry classroom, Ap chemistry What Makes Bases Strong Or Weak A strong base, unlike a weak base, dissociates (separates) completely into ions when it dissolves in water. Any base not listed is a weak base. A weak acid is one that. A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. You can think. What Makes Bases Strong Or Weak.
From exokdjngi.blob.core.windows.net
What Is Meant By Strong Bases And Weak Bases at Vicky Ashby blog What Makes Bases Strong Or Weak Classifying acids or bases as strong or weak has nothing to do with their. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. A base that is a strong electrolyte is called a strong base, while a base that is a weak electrolyte is called a weak base. We are going to use the.. What Makes Bases Strong Or Weak.
From www.expii.com
Strong and Weak Acids and Bases — Definition & Examples Expii What Makes Bases Strong Or Weak A weak acid is one that. There are very few strong bases (table \(\pageindex{1}\)); A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. A strong base, unlike a. What Makes Bases Strong Or Weak.
From www.teachoo.com
Difference between Strong and Weak Base with Examples [in Table] What Makes Bases Strong Or Weak This page explains the terms strong and weak as applied to bases. There are very few strong bases (table \(\pageindex{1}\)); You can think of the compound as. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are. As a. What Makes Bases Strong Or Weak.
From www.slideserve.com
PPT AcidBase PowerPoint Presentation, free download ID853956 What Makes Bases Strong Or Weak A strong acid is one that loses h + easily, meaning that its conjugate base holds the h + weakly and is therefore a weak base. Classifying acids or bases as strong or weak has nothing to do with their. You can think of the compound as. We are going to use the. A base that is a strong electrolyte. What Makes Bases Strong Or Weak.