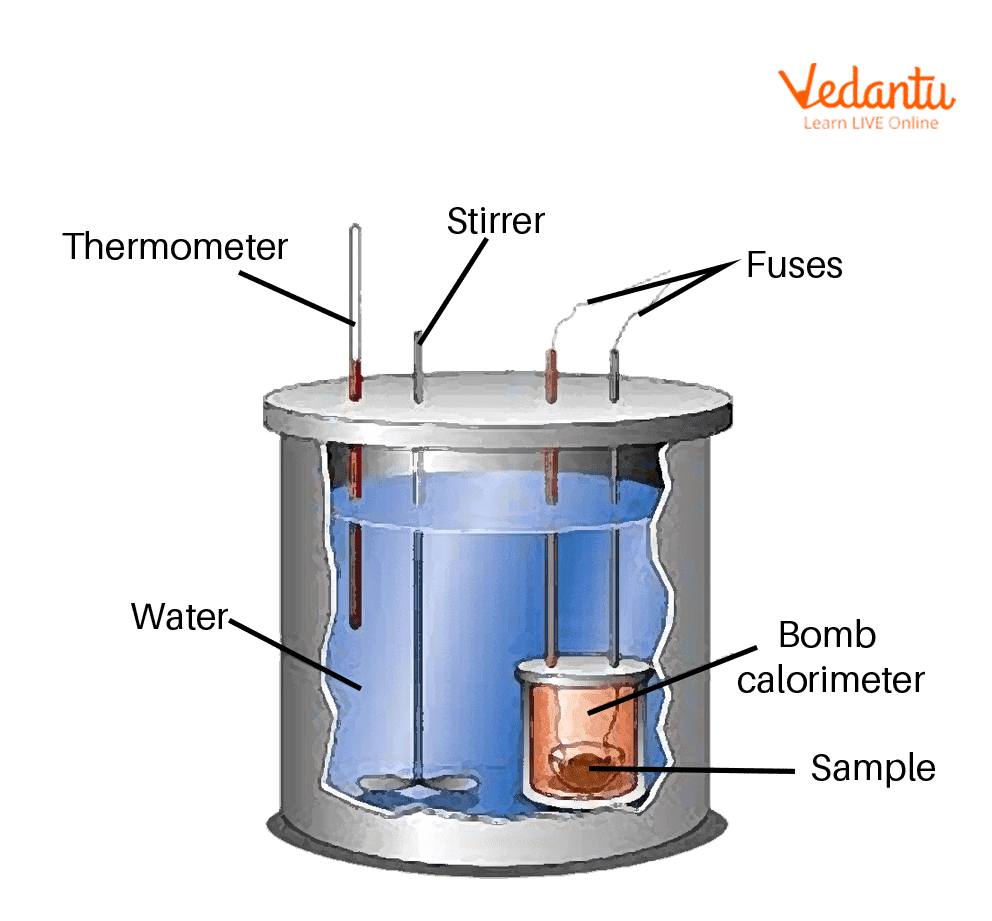
Calorimetric Bomb . environmental scientists possess a secret weapon in their arsenal — the calorimetric bomb. the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. bomb calorimeter formula : Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\).
from www.vedantu.com
the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). environmental scientists possess a secret weapon in their arsenal — the calorimetric bomb. bomb calorimeter formula : the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs.
Bomb Calorimeter Learn Important Terms and Concepts
Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. environmental scientists possess a secret weapon in their arsenal — the calorimetric bomb. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. bomb calorimeter formula : this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\).
From abpdu.lbl.gov
IKA Bomb Calorimeter C2000 Basic ABPDU Calorimetric Bomb The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. environmental scientists possess a secret weapon in their arsenal — the calorimetric bomb. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). Dulong’s. Calorimetric Bomb.
From www.phywe.com
Determination of the enthalpy of combustion with a calorimetric bomb Calorimetric Bomb the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. this value and the measured increase in temperature of. Calorimetric Bomb.
From saylordotorg.github.io
Calorimetry Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\). Calorimetric Bomb.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Calorimetric Bomb environmental scientists possess a secret weapon in their arsenal — the calorimetric bomb. bomb calorimeter formula : The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. this value and the measured increase in temperature of the calorimeter can be used in equation. Calorimetric Bomb.
From www.researchgate.net
Calorimetric bomb 1 locknut; 2 metal ring; 3 flare nut; 4 rubber Calorimetric Bomb The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis. Calorimetric Bomb.
From www.researchgate.net
View of the interior of the calorimetric bomb (1) benzoic acid pellet Calorimetric Bomb The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. environmental scientists possess a secret weapon in their arsenal. Calorimetric Bomb.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. bomb calorimeter formula : this value and the measured increase in temperature of the. Calorimetric Bomb.
From www.scientific.asia
BOMB CALORIMETER Asian Scientific Industries Calorimetric Bomb The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. environmental scientists possess a secret weapon in their arsenal — the calorimetric bomb. the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when. Calorimetric Bomb.
From www.researchgate.net
Calorimetric bomb (Griu and Lunguleasa 2016) Download Scientific Diagram Calorimetric Bomb bomb calorimeter formula : The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. environmental scientists possess a. Calorimetric Bomb.
From bcsmachinery.en.made-in-china.com
Diesel Oil/Fuel Oil Oxygen Calorimetric Bomb (Digital Type) China Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. bomb calorimeter formula : environmental scientists possess a secret weapon in their arsenal —. Calorimetric Bomb.
From www.parrinst.com
Oxygen Bomb Calorimeters Parr Instrument Company Calorimetric Bomb Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. bomb calorimeter formula : The purpose of this research is to determine the effect of. Calorimetric Bomb.
From www.parrinst.com
Oxygen Bomb Calorimeters Parr Instrument Company Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. bomb calorimeter formula : The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. the bomb calorimeter is a laboratory instrument used to measure the amount. Calorimetric Bomb.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimetric Bomb bomb calorimeter formula : the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). environmental scientists possess a secret weapon in their arsenal — the calorimetric bomb. the. Calorimetric Bomb.
From www.diningandcooking.com
Calorimetric Bomb Dining and Cooking Calorimetric Bomb the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. environmental scientists possess a secret weapon in. Calorimetric Bomb.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is. Calorimetric Bomb.
From www.electric-test.com
Bomb Calorimetric Method Calorific Meter Oil Calorific Value Testing Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. bomb calorimeter formula : environmental scientists possess a secret weapon in their arsenal —. Calorimetric Bomb.
From www.chegg.com
Solved 9. Bomb Calorimetric data for the combustion of Calorimetric Bomb The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. environmental scientists possess a secret weapon in their arsenal. Calorimetric Bomb.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetric Bomb Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. bomb calorimeter formula : the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. environmental scientists. Calorimetric Bomb.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetric Bomb Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). The purpose of this research is to determine the effect of using the. Calorimetric Bomb.
From www.alamy.com
Calorimetric bomb Mr Mahler, vintage engraved illustration Stock Photo Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. this value and the measured increase in temperature of the calorimeter can be used in. Calorimetric Bomb.
From www.researchgate.net
Bomb Calorimeter Energy Balance Terms and Definitions Download Table Calorimetric Bomb bomb calorimeter formula : Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. the bomb. Calorimetric Bomb.
From eboardstudy.online
Determination of calorific value of a fuel BOMB CALORIMETER eBoard Study Calorimetric Bomb this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis. Calorimetric Bomb.
From exosbbnfj.blob.core.windows.net
Calorimetry Ap Chemistry at Michael Faust blog Calorimetric Bomb this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. bomb calorimeter formula : environmental scientists possess a secret weapon in their arsenal. Calorimetric Bomb.
From www.youtube.com
Using a Bomb Calorimeter YouTube Calorimetric Bomb the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). Dulong’s formula used to calculate the theoretical calorific value of fuel if the. Calorimetric Bomb.
From www.parrinst.com
Oxygen Bomb Calorimeters Parr Instrument Company Calorimetric Bomb the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. bomb calorimeter formula : the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. this value and the measured increase in temperature of the. Calorimetric Bomb.
From www.elettronicaveneta.com
Determination of the enthalpy of combustion by a calorimetric bomb Calorimetric Bomb Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. environmental scientists possess a secret weapon in their arsenal. Calorimetric Bomb.
From www.engineeringlabchina.com
Bomb Calorimeter Manufacturers, Suppliers & Exporters in China Calorimetric Bomb Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. this value and the measured increase in temperature of the calorimeter can be used in. Calorimetric Bomb.
From www.researchgate.net
Calorimetric bomb 1 locknut; 2 metal ring; 3 flare nut; 4 rubber Calorimetric Bomb The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. environmental scientists possess a secret weapon in their arsenal. Calorimetric Bomb.
From dxobmbkmm.blob.core.windows.net
Bomb Calorimeter Used To Measure at Angela Johnson blog Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value. Calorimetric Bomb.
From exobdylqi.blob.core.windows.net
Bomb Calorimeter What Does It Do at Joann Guarino blog Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. bomb calorimeter formula : environmental scientists possess a secret weapon in their arsenal — the calorimetric bomb. the bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess. Calorimetric Bomb.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimetric Bomb the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\). Calorimetric Bomb.
From www.youtube.com
Bomb Calorimetry Introduction Physical Chemistry Laboratory YouTube Calorimetric Bomb bomb calorimeter formula : Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). environmental scientists possess a secret weapon in. Calorimetric Bomb.
From www.britannica.com
Bomb calorimeter measurement device Britannica Calorimetric Bomb bomb calorimeter formula : this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine \(c_{bomb}\). The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. environmental scientists possess a secret weapon in their arsenal. Calorimetric Bomb.
From www.biovera.com.br
Bomba Calorimétrica IKA C 200 h Biovera Calorimetric Bomb The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of elementary combustibles is known. this value and the measured increase in temperature of. Calorimetric Bomb.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetric Bomb The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. this value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\). Calorimetric Bomb.