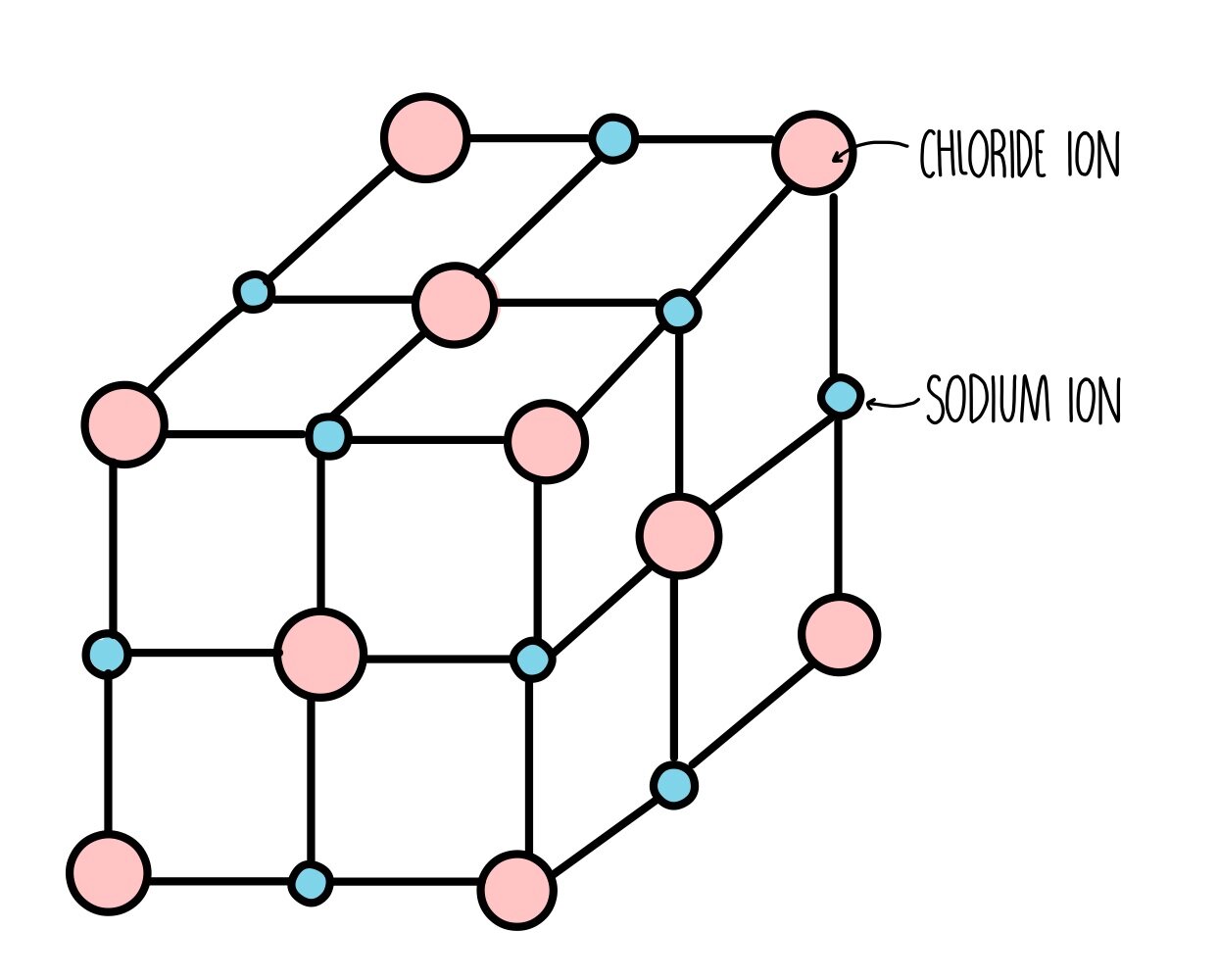
Crystal Compounds Examples . A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Ionic, covalent network, metallic and molecular. There are four main types of crystalline solids: Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. They are distinguished from each other based. Structure refers to the internal arrangement of.
from www.thesciencehive.co.uk
There are four main types of crystalline solids: They are distinguished from each other based. Structure refers to the internal arrangement of. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Ionic, covalent network, metallic and molecular.
Ionic Bonding — the science hive
Crystal Compounds Examples Structure refers to the internal arrangement of. There are four main types of crystalline solids: A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. They are distinguished from each other based. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Ionic, covalent network, metallic and molecular. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Structure refers to the internal arrangement of.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Crystal Compounds Examples There are four main types of crystalline solids: Ionic, covalent network, metallic and molecular. Structure refers to the internal arrangement of. They are distinguished from each other based. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Crystal, any solid material in which the component atoms are arranged in. Crystal Compounds Examples.
From sciencenotes.org
Covalent Compounds Examples and Properties Crystal Compounds Examples They are distinguished from each other based. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Structure refers to the internal arrangement of. Ionic, covalent network, metallic and molecular. There are four main types of crystalline solids: Crystal, any solid material in which the component atoms are arranged in. Crystal Compounds Examples.
From www.britannica.com
Mineral Occurrence, Formation, Compound Britannica Crystal Compounds Examples Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Ionic, covalent network, metallic and molecular. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. There are four main types of crystalline solids: They are distinguished from each. Crystal Compounds Examples.
From chem.libretexts.org
Crystalline Solid Structures Chemistry LibreTexts Crystal Compounds Examples They are distinguished from each other based. Structure refers to the internal arrangement of. There are four main types of crystalline solids: Ionic, covalent network, metallic and molecular. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. A crystal structure is defined as the particular repeating arrangement of atoms. Crystal Compounds Examples.
From eduinput.com
Crystalline solids Properties, types, examples use Crystal Compounds Examples A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Structure refers to the internal arrangement of. They are distinguished from each other based. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Examples of everyday materials you. Crystal Compounds Examples.
From www.slideserve.com
PPT Lecture 3 Rocks and Minerals PowerPoint Presentation, free Crystal Compounds Examples There are four main types of crystalline solids: Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Structure refers to the internal arrangement of. Ionic, covalent network, metallic and molecular. They are distinguished from each other based. Crystal, any solid material in which the component atoms are arranged in. Crystal Compounds Examples.
From www.pinterest.com
The Crystal Lattice Structure of Ionic Compounds infographic diagram Crystal Compounds Examples Ionic, covalent network, metallic and molecular. Structure refers to the internal arrangement of. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. They are distinguished from each other based. There are four main types of crystalline solids: A crystal structure is defined as the particular repeating. Crystal Compounds Examples.
From courses.lumenlearning.com
The Solid State of Matter Chemistry Crystal Compounds Examples They are distinguished from each other based. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. There are four main types of crystalline solids: Structure refers to the internal arrangement of.. Crystal Compounds Examples.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive Crystal Compounds Examples Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Structure refers to the internal arrangement of. There are four main types of crystalline solids: They are distinguished from each other based.. Crystal Compounds Examples.
From chemistryskills.com
Classification of crystals Chemistry Skills Crystal Compounds Examples They are distinguished from each other based. Structure refers to the internal arrangement of. Ionic, covalent network, metallic and molecular. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects. Crystal Compounds Examples.
From www.britannica.com
Cesium chloride chemical compound Britannica Crystal Compounds Examples There are four main types of crystalline solids: Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. They are distinguished from each other based. Ionic, covalent network, metallic and molecular. Crystal,. Crystal Compounds Examples.
From chem.libretexts.org
Introduction to Crystal Field Theory Chemistry LibreTexts Crystal Compounds Examples Ionic, covalent network, metallic and molecular. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. They are distinguished from each other based. There are four main types of crystalline solids: Structure. Crystal Compounds Examples.
From www.slideserve.com
PPT Ionic bondscrystalline structure PowerPoint Presentation, free Crystal Compounds Examples There are four main types of crystalline solids: Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Ionic, covalent network, metallic and molecular. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Structure refers to. Crystal Compounds Examples.
From sciencenotes.org
Ionic Compound Properties Crystal Compounds Examples Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. They are distinguished from each other based. There are four main types of crystalline solids: A crystal. Crystal Compounds Examples.
From courses.lumenlearning.com
Types of Crystals Boundless Chemistry Crystal Compounds Examples Ionic, covalent network, metallic and molecular. They are distinguished from each other based. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Structure refers to the. Crystal Compounds Examples.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Crystal Compounds Examples Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. There are four main types of crystalline solids: A crystal structure is defined as the particular repeating. Crystal Compounds Examples.
From socratic.org
A molecular compound is formed when a chemical reaction occurs between Crystal Compounds Examples Structure refers to the internal arrangement of. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. They are distinguished from each other based. There are four main types of crystalline solids:. Crystal Compounds Examples.
From www.vectorstock.com
Ionic crystals the structure of sodium chloride Vector Image Crystal Compounds Examples Structure refers to the internal arrangement of. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. They are distinguished from each other based. Crystal, any solid material in which the component. Crystal Compounds Examples.
From sciencenotes.org
Examples of Ionic Compounds in Everyday Life Crystal Compounds Examples Structure refers to the internal arrangement of. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. There are four main types of crystalline solids: They are. Crystal Compounds Examples.
From www.researchgate.net
Crystal structures of (a) bodycenteredcubic (bcc), (b) and (c) A15 Crystal Compounds Examples They are distinguished from each other based. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Structure refers to the internal arrangement of. There are four main types of crystalline solids: A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal.. Crystal Compounds Examples.
From studiousguy.com
9 Ionic Bond Examples in Daily Life StudiousGuy Crystal Compounds Examples A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. They are distinguished from each other based. Structure refers to the internal arrangement of. Ionic, covalent network, metallic and molecular. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal. Crystal Compounds Examples.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Crystal Compounds Examples Ionic, covalent network, metallic and molecular. There are four main types of crystalline solids: Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. They are distinguished from each other based. Crystal,. Crystal Compounds Examples.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Crystal Compounds Examples Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Ionic, covalent network, metallic and molecular. Structure refers to the internal arrangement of. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. A crystal structure is. Crystal Compounds Examples.
From courses.lumenlearning.com
Compounds Essential to Human Functioning Anatomy and Crystal Compounds Examples Structure refers to the internal arrangement of. They are distinguished from each other based. There are four main types of crystalline solids: A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects. Crystal Compounds Examples.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples Crystal Compounds Examples A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Structure refers to the internal arrangement of. Ionic, covalent network, metallic and molecular. They are distinguished from each other based. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal. Crystal Compounds Examples.
From www.geologyin.com
Crystal Structure and Crystal Systems Crystal Compounds Examples Structure refers to the internal arrangement of. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Crystal, any solid material in which the component atoms are arranged in a definite pattern. Crystal Compounds Examples.
From sciencenotes.org
Compounds With Both Ionic and Covalent Bonds Crystal Compounds Examples Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. There are four main types of crystalline solids: Ionic, covalent network, metallic and molecular. They are distinguished from each other based. Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals),. Crystal Compounds Examples.
From www.beautifulchemistry.net
Crystal Structure — Beautiful Chemistry Crystal Compounds Examples Ionic, covalent network, metallic and molecular. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. There are four main types of crystalline solids: Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Structure refers to. Crystal Compounds Examples.
From www.britannica.com
compound Definition & Examples Britannica Crystal Compounds Examples Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Ionic, covalent network, metallic and molecular. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. There are four main types of crystalline solids: Structure refers to. Crystal Compounds Examples.
From bernieralice.hyperphp.com
Types Of Crystal Lattice Structure bernieralice Crystal Compounds Examples Ionic, covalent network, metallic and molecular. Structure refers to the internal arrangement of. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. They are distinguished from each other based. There are four main types of crystalline solids: Crystal, any solid material in which the component atoms are arranged in a definite. Crystal Compounds Examples.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Crystal Compounds Examples A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. They are distinguished from each other based. There are four main types of crystalline solids: Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Examples of everyday materials. Crystal Compounds Examples.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation, free Crystal Compounds Examples Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. They are distinguished from each other based. There are four main types of crystalline solids: Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Ionic, covalent. Crystal Compounds Examples.
From www.slideserve.com
PPT Mineral/Crystal Chemistry and Classification of Minerals Crystal Compounds Examples They are distinguished from each other based. Structure refers to the internal arrangement of. There are four main types of crystalline solids: Examples of everyday materials you encounter as crystals are table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes. Ionic, covalent network, metallic and molecular. A crystal structure is defined as the particular repeating arrangement of atoms. Crystal Compounds Examples.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Crystal Compounds Examples They are distinguished from each other based. There are four main types of crystalline solids: Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. Ionic, covalent network, metallic. Crystal Compounds Examples.
From www.britannica.com
Metallic bond Properties, Examples, & Explanation Britannica Crystal Compounds Examples Structure refers to the internal arrangement of. A crystal structure is defined as the particular repeating arrangement of atoms (molecules or ions) throughout a crystal. There are four main types of crystalline solids: Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. They are distinguished from. Crystal Compounds Examples.