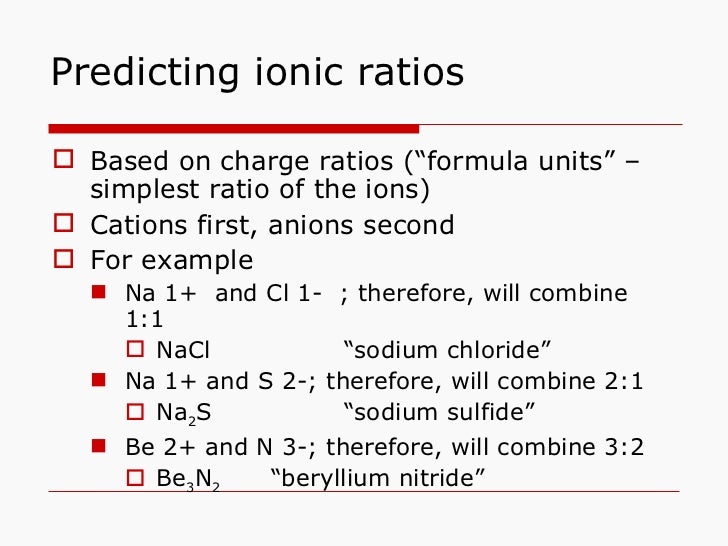
Ionic Compound Ratio . The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. 3.3 formulas for ionic compounds. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Write the chemical formula for a simple ionic compound. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. Although both of these ions have higher charges. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal.
from www.slideshare.net
Although both of these ions have higher charges. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. 3.3 formulas for ionic compounds. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Write the chemical formula for a simple ionic compound.
Ch 8 ionic compounds
Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. Although both of these ions have higher charges. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. 3.3 formulas for ionic compounds. Write the chemical formula for a simple ionic compound. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative.
From lessonlibrarybumgarner.z13.web.core.windows.net
Ionic Compound Nomenclature Worksheet Answers Ionic Compound Ratio 3.3 formulas for ionic compounds. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Write the chemical formula for a simple ionic compound. The formula of an. Ionic Compound Ratio.
From in.pinterest.com
Naming Compounds Worksheet, Polyatomic Ion, Ionic Compound, Ionic Ionic Compound Ratio Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Write the chemical formula for a simple ionic compound. The formula of an ionic compound must have a ratio. Ionic Compound Ratio.
From askfilo.com
Radius ratio of an ionic compound is 0.93. The structure of the above io.. Ionic Compound Ratio In covalent compounds, electrons are shared between bonded atoms and are simultaneously. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula. Ionic Compound Ratio.
From www.pathwaystochemistry.com
Naming Simple Ionic Compounds Pathways to Chemistry Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. Write the chemical formula for a simple ionic compound. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Although both of these ions have higher charges. 3.3 formulas for ionic compounds. The formula of. Ionic Compound Ratio.
From www.youtube.com
Naming and Writing Formulas for Ionic Compounds YouTube Ionic Compound Ratio 3.3 formulas for ionic compounds. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Write the chemical formula for a simple ionic compound. When an ionic compound is formed from magnesium and oxygen,. Ionic Compound Ratio.
From www.myxxgirl.com
Lewis Structure Of Ionic Compound My XXX Hot Girl Ionic Compound Ratio The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and. Ionic Compound Ratio.
From saylordotorg.github.io
Chemical Compounds Ionic Compound Ratio Although both of these ions have higher charges. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. Write the chemical formula. Ionic Compound Ratio.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Although both of these ions have higher charges. The formula of an. Ionic Compound Ratio.
From utedzz.blogspot.com
Periodic Table Ions List Periodic Table Timeline Ionic Compound Ratio Write the chemical formula for a simple ionic compound. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. Although both of these ions have higher charges. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. 3.3 formulas for ionic compounds. The formula of. Ionic Compound Ratio.
From www.housview.com
Collection Of Ionic Compounds Naming Worksheet Free Worksheets Samples Ionic Compound Ratio When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Although both of these ions have higher charges. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. The formula of an ionic compound represents the simplest ratio. Ionic Compound Ratio.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The formula of an ionic compound must have a ratio of ions such. Ionic Compound Ratio.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Ionic Compound Ratio In covalent compounds, electrons are shared between bonded atoms and are simultaneously. 3.3 formulas for ionic compounds. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Although both of these ions have higher charges. The formula of an ionic compound represents the simplest ratio. Ionic Compound Ratio.
From chem.libretexts.org
2.7 Ions and Ionic Compounds Chemistry LibreTexts Ionic Compound Ratio When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. The formula of an ionic compound must have a ratio of ions such that the numbers of positive. Ionic Compound Ratio.
From www.slideserve.com
PPT Chapter 11 Chemical Bonds PowerPoint Presentation, free download Ionic Compound Ratio Although both of these ions have higher charges. Write the chemical formula for a simple ionic compound. 3.3 formulas for ionic compounds. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The formula of an ionic compound represents the lowest whole number ratio of. Ionic Compound Ratio.
From www.nagwa.com
Question Video Understanding How to Represent the Crystal Structure of Ionic Compound Ratio The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. Write the chemical formula for a simple ionic compound. When an ionic compound. Ionic Compound Ratio.
From docslib.org
Solubility of Ionic Compound DocsLib Ionic Compound Ratio When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. The formula of an ionic compound must have a ratio of ions such. Ionic Compound Ratio.
From aunitedkingdomfilm.com
Determine Whether The Following Pairs Of Elements Can Form Ionic Compounds Ionic Compound Ratio Although both of these ions have higher charges. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. Write the chemical formula for a simple ionic compound. 3.3 formulas for ionic compounds. Ionic compounds contain both cations and anions in a ratio that results in no net. Ionic Compound Ratio.
From www.slideserve.com
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free Ionic Compound Ratio The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. 3.3 formulas for ionic compounds. The formula of an ionic compound. Ionic Compound Ratio.
From www.compoundworksheets.com
39 Writing Formulas Ionic Compounds Worksheet Answers Combining Like Ionic Compound Ratio When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. 3.3 formulas for ionic compounds. Although both of these ions have. Ionic Compound Ratio.
From askfilo.com
In the ionic compound AB the ratio rA+ rB− is 0.414. Indicate the correc.. Ionic Compound Ratio When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound must have a ratio of ions. Ionic Compound Ratio.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. The formula of an ionic compound must have a ratio of ions such that the. Ionic Compound Ratio.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 Ionic Compound Ratio The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. 3.3 formulas for ionic compounds. Write the chemical formula for a simple ionic compound. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Ionic compounds contain both cations and anions in a ratio. Ionic Compound Ratio.
From www.ck12.org
Ionic Compounds CK12 Foundation Ionic Compound Ratio When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. The formula of an ionic compound must have a ratio of ions such. Ionic Compound Ratio.
From slideplayer.com
Ionic Compounds. ppt download Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. In covalent compounds, electrons are shared between bonded atoms and are simultaneously.. Ionic Compound Ratio.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. Although both of these ions have higher charges. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The formula of an ionic. Ionic Compound Ratio.
From www.slideshare.net
Ch 8 ionic compounds Ionic Compound Ratio Write the chemical formula for a simple ionic compound. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. When an ionic compound. Ionic Compound Ratio.
From study.com
What Are Ionic Compounds? Definition, Examples & Reactions Video Ionic Compound Ratio Although both of these ions have higher charges. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. 3.3 formulas for ionic compounds. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. In covalent compounds, electrons are shared between bonded. Ionic Compound Ratio.
From slideplayer.com
Unit 2 Chapter 5 Ionic Compound Names ppt download Ionic Compound Ratio 3.3 formulas for ionic compounds. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The formula of an ionic compound. Ionic Compound Ratio.
From study.com
Chemical Formula for Ionic Compound Binary & Polyatomic Lesson Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. The formula of an ionic compound represents the. Ionic Compound Ratio.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Ionic Compound Ratio 3.3 formulas for ionic compounds. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Write the chemical formula for a simple ionic compound.. Ionic Compound Ratio.
From www.slideshare.net
Ch 8 ionic compounds Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. 3.3 formulas for ionic compounds. Write the chemical formula for a simple ionic compound. The formula of an ionic compound must have a ratio of. Ionic Compound Ratio.
From www.slideserve.com
PPT Naming Binary Ionic Compounds PowerPoint Presentation, free Ionic Compound Ratio The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. Write the chemical formula for a simple ionic compound. The formula of an ionic. Ionic Compound Ratio.
From www.youtube.com
Writing Chemical Formulas For Ionic Compounds YouTube Ionic Compound Ratio Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The formula of an ionic compound. Ionic Compound Ratio.
From www.youtube.com
Ionic Compound Ion Ratios YouTube Ionic Compound Ratio 3.3 formulas for ionic compounds. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal. The formula of an ionic compound represents the lowest. Ionic Compound Ratio.
From www.revision.my
5.7.1 Properties of Ionic and Covalent Compounds Revision.my Ionic Compound Ratio The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Write the chemical formula for a simple ionic compound. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The formula of an. Ionic Compound Ratio.