Calorimetry Of Water . we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. It stops once thermal equilibrium between the pan and the water is. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. heat transfer restores thermal equilibrium once the water and pan are in contact; It can analyze the heat exchange between up to 3 objects. the calorimetry calculator can help you solve complex calorimetry problems. liquid water has one of the highest specific heats known.
from www.tec-science.com
heat transfer restores thermal equilibrium once the water and pan are in contact; To do so, the heat is exchanged with a. liquid water has one of the highest specific heats known. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. It can analyze the heat exchange between up to 3 objects. the calorimetry calculator can help you solve complex calorimetry problems. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. calorimetry is used to measure amounts of heat transferred to or from a substance. It stops once thermal equilibrium between the pan and the water is.
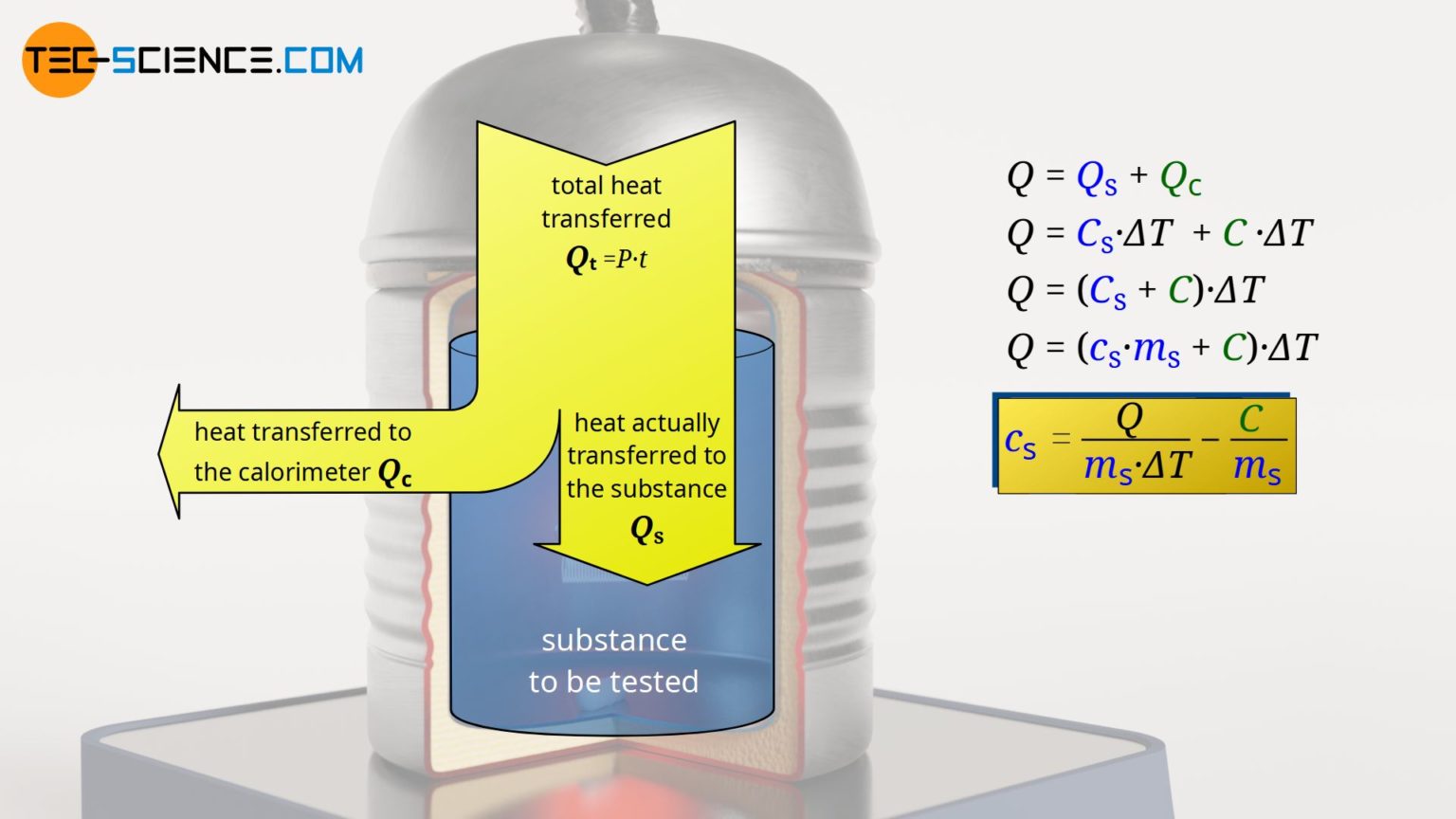
Calorimeter to determine the specific heat capacities of liquids tec
Calorimetry Of Water To do so, the heat is exchanged with a. the calorimetry calculator can help you solve complex calorimetry problems. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. It stops once thermal equilibrium between the pan and the water is. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. It can analyze the heat exchange between up to 3 objects. liquid water has one of the highest specific heats known. heat transfer restores thermal equilibrium once the water and pan are in contact;
From byjus.com
Hundred gram of water at 40 degree celsius is contained in a Calorimetry Of Water It stops once thermal equilibrium between the pan and the water is. heat transfer restores thermal equilibrium once the water and pan are in contact; the calorimetry calculator can help you solve complex calorimetry problems. To do so, the heat is exchanged with a. liquid water has one of the highest specific heats known. calorimetry is. Calorimetry Of Water.
From byjus.com
In a calorimeter (water equivalent=40 g) are 200 g of water and 50 g of Calorimetry Of Water To do so, the heat is exchanged with a. It stops once thermal equilibrium between the pan and the water is. It can analyze the heat exchange between up to 3 objects. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. liquid water has one of. Calorimetry Of Water.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Calorimetry Of Water It stops once thermal equilibrium between the pan and the water is. the calorimetry calculator can help you solve complex calorimetry problems. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. It can analyze the heat exchange between up to 3 objects. calorimetry is used to measure amounts of heat. Calorimetry Of Water.
From www.physicsbootcamp.org
Calorimetry Calorimetry Of Water To do so, the heat is exchanged with a. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. It can analyze the heat exchange between up to 3 objects. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. the calorimetry calculator can. Calorimetry Of Water.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Of Water To do so, the heat is exchanged with a. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. calorimetry is used to measure amounts of heat transferred to or from a substance. . Calorimetry Of Water.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Of Water liquid water has one of the highest specific heats known. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. It stops once thermal equilibrium between the pan and the water is. once. Calorimetry Of Water.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimetry Of Water It stops once thermal equilibrium between the pan and the water is. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. the calorimetry calculator can help you solve complex calorimetry problems. liquid water has one of the highest specific heats known. heat transfer restores. Calorimetry Of Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Of Water To do so, the heat is exchanged with a. heat transfer restores thermal equilibrium once the water and pan are in contact; before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. It can analyze the heat exchange between up to 3 objects. calorimetry is used to measure amounts of heat. Calorimetry Of Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Of Water It can analyze the heat exchange between up to 3 objects. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. calorimetry is used to measure amounts of heat transferred to or from a substance. liquid water has one of the highest specific heats known. It stops once thermal equilibrium. Calorimetry Of Water.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Of Water heat transfer restores thermal equilibrium once the water and pan are in contact; calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an.. Calorimetry Of Water.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Of Water the calorimetry calculator can help you solve complex calorimetry problems. liquid water has one of the highest specific heats known. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. heat transfer restores thermal equilibrium once the water and pan are in contact; It can analyze the heat exchange between. Calorimetry Of Water.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Calorimetry Of Water once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. It stops once thermal equilibrium between the pan and the water is. liquid water has one of the highest. Calorimetry Of Water.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Of Water calorimetry is used to measure amounts of heat transferred to or from a substance. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. To do so, the heat is exchanged with a. liquid water has one of the highest specific heats known. we will. Calorimetry Of Water.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry Of Water the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. To do so, the heat is exchanged with a. before we practice calorimetry problems involving. Calorimetry Of Water.
From saylordotorg.github.io
Calorimetry Calorimetry Of Water To do so, the heat is exchanged with a. It stops once thermal equilibrium between the pan and the water is. liquid water has one of the highest specific heats known. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. heat transfer restores thermal equilibrium. Calorimetry Of Water.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Of Water before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. heat transfer restores thermal equilibrium once the water and pan are in contact; To do so, the heat is exchanged with a. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\). Calorimetry Of Water.
From exyqlxbwd.blob.core.windows.net
Calorimetry Fundamentals Instrumentation And Applications at Jeffrey Calorimetry Of Water To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up. Calorimetry Of Water.
From wisc.pb.unizin.org
Day 27 Thermochemistry and Enthalpy Chemistry 109, Fall 2020 Calorimetry Of Water It can analyze the heat exchange between up to 3 objects. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. liquid water has one of the highest specific heats known. the calorimetry. Calorimetry Of Water.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Of Water To do so, the heat is exchanged with a. It stops once thermal equilibrium between the pan and the water is. the calorimetry calculator can help you solve complex calorimetry problems. liquid water has one of the highest specific heats known. It can analyze the heat exchange between up to 3 objects. once the “water equivalent” is. Calorimetry Of Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimetry Of Water calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat exchange between up to 3 objects. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. To do so, the heat is exchanged with a. liquid water. Calorimetry Of Water.
From exykjnxoy.blob.core.windows.net
Calorimetry Lab Biology at Tina Powers blog Calorimetry Of Water before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. calorimetry is used to measure amounts of heat transferred to or from a substance. It stops once thermal equilibrium between the pan and the water is. once the “water equivalent” is determined for a calorimeter, the temperature change can be used. Calorimetry Of Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Of Water heat transfer restores thermal equilibrium once the water and pan are in contact; To do so, the heat is exchanged with a. It stops once thermal equilibrium between the pan and the water is. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. we will apply calorimetry to two different. Calorimetry Of Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Of Water before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. It stops once thermal equilibrium between the pan and the water is. It can analyze the heat exchange between up to 3 objects. liquid water has one of the highest specific heats known. once the “water equivalent” is determined for a. Calorimetry Of Water.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Of Water once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. heat transfer restores thermal equilibrium once the water and pan are in contact; To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance.. Calorimetry Of Water.
From www.youtube.com
How to find calorimeter constant YouTube Calorimetry Of Water the calorimetry calculator can help you solve complex calorimetry problems. It stops once thermal equilibrium between the pan and the water is. It can analyze the heat exchange between up to 3 objects. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. calorimetry is used to measure amounts of. Calorimetry Of Water.
From www.chegg.com
Solved A calorimetry contains 150 g of water at 24.6∘C. A Calorimetry Of Water It can analyze the heat exchange between up to 3 objects. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. heat transfer restores thermal equilibrium once the water and pan are in contact; the calorimetry calculator can help you solve complex calorimetry problems. It stops once thermal equilibrium between the. Calorimetry Of Water.
From www.youtube.com
Calorimetry Lecture 2 Thermal Physics Water Equivalent of Substance Law Calorimetry Of Water heat transfer restores thermal equilibrium once the water and pan are in contact; calorimetry is used to measure amounts of heat transferred to or from a substance. liquid water has one of the highest specific heats known. It stops once thermal equilibrium between the pan and the water is. the calorimetry calculator can help you solve. Calorimetry Of Water.
From www.youtube.com
Calorimetry of NaOH in Water YouTube Calorimetry Of Water It stops once thermal equilibrium between the pan and the water is. liquid water has one of the highest specific heats known. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. It can. Calorimetry Of Water.
From fyoveqkgi.blob.core.windows.net
Examples Of Calorimeters at Joseph Sharp blog Calorimetry Of Water calorimetry is used to measure amounts of heat transferred to or from a substance. It stops once thermal equilibrium between the pan and the water is. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. heat transfer restores thermal equilibrium once the water and pan. Calorimetry Of Water.
From www.toppr.com
In a calorimeter of water equivalent 20g ,water of mass 1.1 kg is taken Calorimetry Of Water we will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. the calorimetry calculator can help you solve complex calorimetry problems. It stops once thermal equilibrium between the. Calorimetry Of Water.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Calorimetry Of Water It can analyze the heat exchange between up to 3 objects. calorimetry is used to measure amounts of heat transferred to or from a substance. heat transfer restores thermal equilibrium once the water and pan are in contact; the calorimetry calculator can help you solve complex calorimetry problems. before we practice calorimetry problems involving chemical reactions,. Calorimetry Of Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Of Water To do so, the heat is exchanged with a. heat transfer restores thermal equilibrium once the water and pan are in contact; liquid water has one of the highest specific heats known. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. we will apply. Calorimetry Of Water.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimetry Of Water liquid water has one of the highest specific heats known. once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. calorimetry is used to measure amounts of heat transferred to or from a substance. heat transfer restores thermal equilibrium once the water and pan are. Calorimetry Of Water.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Of Water heat transfer restores thermal equilibrium once the water and pan are in contact; once the “water equivalent” is determined for a calorimeter, the temperature change can be used to find \(\delta u_c\) for an. To do so, the heat is exchanged with a. we will apply calorimetry to two different thermodynamic processes, that of transference of heat. Calorimetry Of Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimetry Of Water To do so, the heat is exchanged with a. before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core. It can analyze the heat exchange between up to 3 objects. liquid water has one of the highest specific heats known. we will apply calorimetry to two different thermodynamic processes, that of. Calorimetry Of Water.