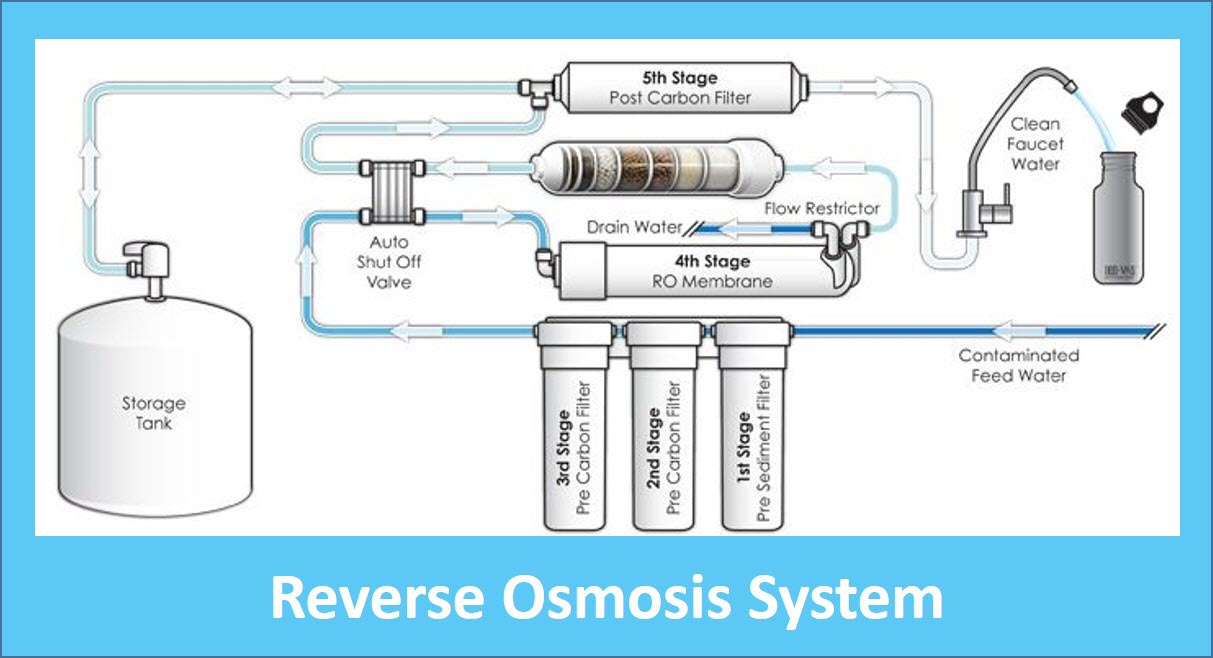
What Is Water Ro . Large molecules (solute) can't cross the membrane, so they remain on one side. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Ro systems are used in a variety of. This type of water filter consists of several filters: Particles, molecules, and ions that are larger than. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane.
from cannonwater.com
Large molecules (solute) can't cross the membrane, so they remain on one side. Particles, molecules, and ions that are larger than. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Ro systems are used in a variety of. This type of water filter consists of several filters: With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system.
A Brief Discussion on Reverse Osmosis (RO) System Cannon Water Technology
What Is Water Ro Ro systems are used in a variety of. This type of water filter consists of several filters: Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Particles, molecules, and ions that are larger than. Ro systems are used in a variety of. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Large molecules (solute) can't cross the membrane, so they remain on one side.
From www.aosbath.com
Benefits of RO Water AOS Bath Singapore What Is Water Ro Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Particles, molecules, and ions that are larger than. Large molecules (solute) can't cross the membrane, so they remain on one side. Ro systems are used in a variety of. With this guide, learn about what reverse osmosis filtration is and the pros and cons. What Is Water Ro.
From www.water-rightgroup.com
Benefits of Drinking Reverse Osmosis Water WaterRight What Is Water Ro This type of water filter consists of several filters: With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Ro systems are used in a variety of. Particles, molecules, and ions that are larger than. Reverse osmosis or ro is a filtration method that is used to. What Is Water Ro.
From www.walmart.com
Express Water Reverse Osmosis Water Filtration System 5 Stage RO What Is Water Ro With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Large molecules (solute) can't cross the membrane, so they remain on one side. Particles, molecules, and ions that are larger than. This type of water filter consists of several filters: Reverse osmosis ro water is drinking water. What Is Water Ro.
From www.aqualiteus.com
Everything You Need to Know About Reverse Osmosis Water Filtration What Is Water Ro With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Ro systems are used in a variety of. Large molecules (solute) can't cross the membrane, so they remain on one side.. What Is Water Ro.
From www.hinada.com
1000LPH Purification RO System Hinada What Is Water Ro Large molecules (solute) can't cross the membrane, so they remain on one side. This type of water filter consists of several filters: Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your. What Is Water Ro.
From animalia-life.club
Reverse Osmosis Water Filter What Is Water Ro Ro systems are used in a variety of. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Particles, molecules, and ions that are larger than. Large molecules (solute) can't cross the membrane, so they remain on one side. This type of water filter consists of several. What Is Water Ro.
From netsolwater.com
What is the role of Reverse Osmosis in drinking water treatment What Is Water Ro Ro systems are used in a variety of. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. This type of water filter consists of several filters: Particles, molecules, and ions that are larger than. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis. What Is Water Ro.
From purewatersystems.com.au
What is Reverse Osmosis Water Purification? & Pure Water Systems What Is Water Ro Large molecules (solute) can't cross the membrane, so they remain on one side. Particles, molecules, and ions that are larger than. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Ro systems are used in. What Is Water Ro.
From xajufu.en.made-in-china.com
Hot Selling 500 Liters Per Hour Water RO System Reverse Osmosis Filter What Is Water Ro Particles, molecules, and ions that are larger than. This type of water filter consists of several filters: Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Large molecules (solute) can't. What Is Water Ro.
From aquapure.org.pk
Domestic RO Plant ProSeries 6 Stage System AQUA PURE What Is Water Ro Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Particles, molecules, and ions that are larger than. Ro systems are used in a variety of. This type of water filter consists of several filters: With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis. What Is Water Ro.
From www.kgnglobal.com
Water Treatment KGN Global What Is Water Ro This type of water filter consists of several filters: With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side. What Is Water Ro.
From hardsoftwater.com
RO water vs DI water, which is better for you? Hard and Soft Water What Is Water Ro This type of water filter consists of several filters: With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Ro systems are used in a variety of. Particles, molecules, and ions. What Is Water Ro.
From www.filterwater.com
How Reverse Osmosis Process Works What Is Water Ro With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Reverse osmosis ro. What Is Water Ro.
From pacificwater.com.au
REVERSE OSMOSIS BRACKISH WATER UNITS Pacific Water What Is Water Ro This type of water filter consists of several filters: Large molecules (solute) can't cross the membrane, so they remain on one side. Ro systems are used in a variety of. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Particles, molecules, and ions that are larger. What Is Water Ro.
From www.desertcart.ae
Buy KENT Pearl RO Water Purifier 4 Years Free Service ISI Marked What Is Water Ro Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. This type of water filter consists of several filters: Particles, molecules, and ions that are larger than. Reverse osmosis ro water is drinking water that has. What Is Water Ro.
From netsolwater.com
What are the best solutions to dispose of RO Concentrated water What Is Water Ro Particles, molecules, and ions that are larger than. Ro systems are used in a variety of. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Reverse osmosis or ro is. What Is Water Ro.
From isingsculligan.com
Learn What Is Reverse Osmosis And Reverse Osmosis Systems What Is Water Ro This type of water filter consists of several filters: Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Ro systems are used in a variety of. Large molecules (solute) can't cross the membrane, so they. What Is Water Ro.
From sensorex.com
What is A Reverse Osmosis Water System and How Can You Manage It Sensorex What Is Water Ro Ro systems are used in a variety of. This type of water filter consists of several filters: Particles, molecules, and ions that are larger than. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Large. What Is Water Ro.
From manualwiringimpeller.z14.web.core.windows.net
Reverse Osmosis Process Pdf What Is Water Ro Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Ro systems are used in a variety of. This type of water filter consists of several filters: Large molecules (solute) can't cross the membrane, so they remain on one side. Reverse osmosis or ro is a filtration method that is used to remove ions. What Is Water Ro.
From www.pinterest.com
Do I Need a Whole House Reverse Osmosis System? Fresh Water Systems What Is Water Ro With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Large molecules (solute) can't cross the membrane, so they remain on one side. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the. What Is Water Ro.
From www.sciencefacts.net
Reverse Osmosis Definition, Principle, and Applications What Is Water Ro Ro systems are used in a variety of. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Particles, molecules, and ions that are larger than. This type of water filter consists of several filters: Reverse osmosis ro water is drinking water that has been filtered in. What Is Water Ro.
From www.hitechro.net
HiTech best water purifier, domestic, commercial and industrial What Is Water Ro This type of water filter consists of several filters: Particles, molecules, and ions that are larger than. Large molecules (solute) can't cross the membrane, so they remain on one side. Ro systems are used in a variety of. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Reverse osmosis or ro is a. What Is Water Ro.
From quenchwater.com
What is Reverse Osmosis and How Does it Work? What Is Water Ro Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Particles, molecules, and ions that are larger than. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Large molecules. What Is Water Ro.
From nationalwaterservice.com
Manuals & Brochures National Water Service What Is Water Ro With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Particles, molecules, and ions that are larger than. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. This type of water filter consists of several filters: Ro systems are used. What Is Water Ro.
From www.artstation.com
ArtStation Water Ro Poster What Is Water Ro This type of water filter consists of several filters: Ro systems are used in a variety of. Particles, molecules, and ions that are larger than. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis. What Is Water Ro.
From www.walmart.com
Olympia Water Systems 5Stage Reverse Osmosis Water Filtration System What Is Water Ro Large molecules (solute) can't cross the membrane, so they remain on one side. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Particles, molecules, and ions that are larger than. With this guide, learn about. What Is Water Ro.
From worldwaterreserve.com
The 10 Best Reverse Osmosis Systems for Home Use [2020] World Water What Is Water Ro This type of water filter consists of several filters: Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Ro systems are used in a variety of. Large molecules (solute) can't cross the membrane, so they remain on one side. Particles, molecules, and ions that are larger than. Reverse osmosis or ro is a. What Is Water Ro.
From cannonwater.com
A Brief Discussion on Reverse Osmosis (RO) System Cannon Water Technology What Is Water Ro Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. This type of water filter consists of several filters: Large. What Is Water Ro.
From industrialinfo.com.my
What is RO Water? Industrial Info Malaysia What Is Water Ro With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. This type of water filter consists of several filters: Particles, molecules, and ions that are larger than. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by. What Is Water Ro.
From www.appliedmembranes.com
5 Stage Reverse Osmosis System Point of Use Home RO Water Filter What Is Water Ro Large molecules (solute) can't cross the membrane, so they remain on one side. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Ro systems are used in a variety of. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules. What Is Water Ro.
From www.123filter.com
iSpring CRO1000 4Stage Tankless Commercial Reverse Osmosis Drinking What Is Water Ro Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Ro systems are used in a variety of. Large molecules (solute) can't cross the membrane, so they remain on one side. With this guide, learn about. What Is Water Ro.
From complete-water.com
What is Reverse Osmosis (RO) and How Does It Work? What Is Water Ro Large molecules (solute) can't cross the membrane, so they remain on one side. Particles, molecules, and ions that are larger than. With this guide, learn about what reverse osmosis filtration is and the pros and cons of having reverse osmosis water in your home. Reverse osmosis or ro is a filtration method that is used to remove ions and molecules. What Is Water Ro.
From www.atlanticbluewaterservices.com
HOW DO REVERSE OSMOSIS DRINKING WATER SYSTEMS WORK? Atlantic Blue What Is Water Ro Ro systems are used in a variety of. Particles, molecules, and ions that are larger than. Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Large molecules (solute) can't cross the membrane, so they remain on one side. Reverse osmosis or ro is a filtration method that is used to remove ions and. What Is Water Ro.
From netsolwater.com
What is the difference between RO water and Mineral water What Is Water Ro Large molecules (solute) can't cross the membrane, so they remain on one side. This type of water filter consists of several filters: Reverse osmosis ro water is drinking water that has been filtered in a reverse osmosis system. Particles, molecules, and ions that are larger than. With this guide, learn about what reverse osmosis filtration is and the pros and. What Is Water Ro.
From www.amazon.in
WaterQ RO25 LPH Commercial RO Water Purifier, 25 Litre (White) 1 Pcs What Is Water Ro Reverse osmosis or ro is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane. Particles, molecules, and ions that are larger than. Large molecules (solute) can't cross the membrane, so they remain on one side. With this guide, learn about. What Is Water Ro.