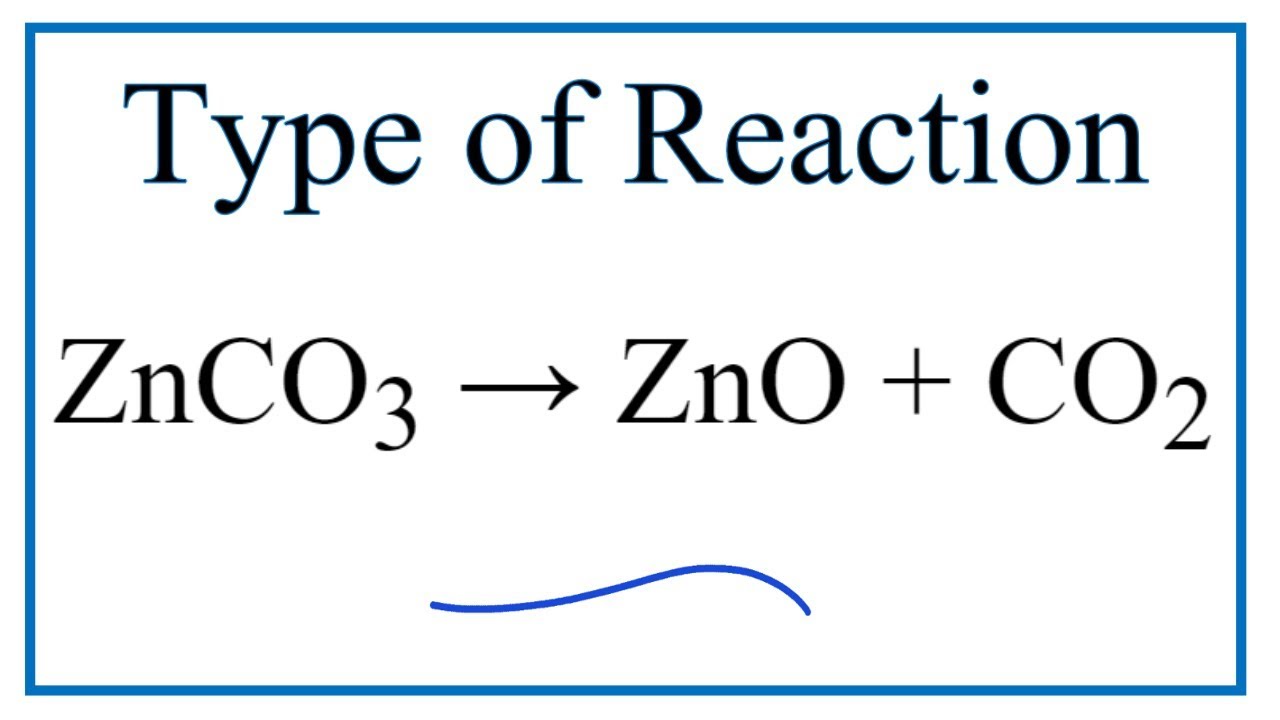
Zinc Carbonate Decomposition Reaction . The compound named zinc carbonate undergoes decomposition in the presence of heat to form. Make sure you know the observations for the decomposition for copper (ii) carbonate. Copper carbonate decomposes at 290 °c. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. Reaction of zinc carbonate upon heating: The aim of this experiment is to compare the reactivity of some different metal carbonates. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Some carbonates are more reactive than others. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. Cuco3 (s) → cuo (s) + co2 (g) examiner tip. Metal carbonates decompose when heated. It turns from green to black when heated. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c.
from www.youtube.com
Copper carbonate decomposes at 290 °c. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Reaction of zinc carbonate upon heating: Metal carbonates decompose when heated. It turns from green to black when heated. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. Make sure you know the observations for the decomposition for copper (ii) carbonate. The aim of this experiment is to compare the reactivity of some different metal carbonates.
Type of Reaction for ZnCO3 = ZnO + CO2 YouTube
Zinc Carbonate Decomposition Reaction Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Reaction of zinc carbonate upon heating: The aim of this experiment is to compare the reactivity of some different metal carbonates. The compound named zinc carbonate undergoes decomposition in the presence of heat to form. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. It turns from green to black when heated. Some carbonates are more reactive than others. Make sure you know the observations for the decomposition for copper (ii) carbonate. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. Copper carbonate decomposes at 290 °c. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Cuco3 (s) → cuo (s) + co2 (g) examiner tip. Metal carbonates decompose when heated. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at.
From www.youtube.com
of Sodium carbonate (balancing equation) YouTube Zinc Carbonate Decomposition Reaction The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. Some carbonates are more reactive than others. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
7.5.2 of Metal Carbonates YouTube Zinc Carbonate Decomposition Reaction The compound named zinc carbonate undergoes decomposition in the presence of heat to form. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. The reaction of basic zinc carbonate. Zinc Carbonate Decomposition Reaction.
From klankxlvf.blob.core.windows.net
Zinc Carbonate Gives Zinc Oxide at Lilian Bigham blog Zinc Carbonate Decomposition Reaction Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. The aim of this experiment is to compare the reactivity of some different metal carbonates. Copper carbonate decomposes at 290 °c. The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. Make sure you know the observations. Zinc Carbonate Decomposition Reaction.
From www.numerade.com
12.5g of zinc carbonate is heated, it to make 8.1g of zinc Zinc Carbonate Decomposition Reaction It turns from green to black when heated. The compound named zinc carbonate undergoes decomposition in the presence of heat to form. Metal carbonates decompose when heated. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor. Zinc Carbonate Decomposition Reaction.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Zinc Carbonate Decomposition Reaction Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Reaction of zinc carbonate upon heating: The compound named zinc carbonate undergoes decomposition in the presence of heat to form. Copper carbonate decomposes at 290 °c. It turns from green to black when heated. Results showed that zinc carbonate hydroxide decomposition started at about. Zinc Carbonate Decomposition Reaction.
From www.toppr.com
2. A metal carbonate according to following reaction M,CO3(s Zinc Carbonate Decomposition Reaction Reaction of zinc carbonate upon heating: Metal carbonates decompose when heated. Cuco3 (s) → cuo (s) + co2 (g) examiner tip. Some carbonates are more reactive than others. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Copper carbonate decomposes at 290 °c. It turns from green to black. Zinc Carbonate Decomposition Reaction.
From www.slideshare.net
reaction Zinc Carbonate Decomposition Reaction Metal carbonates decompose when heated. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form. Zinc Carbonate Decomposition Reaction.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Carbonate Decomposition Reaction The aim of this experiment is to compare the reactivity of some different metal carbonates. Reaction of zinc carbonate upon heating: Some carbonates are more reactive than others. Make sure you know the observations for the decomposition for copper (ii) carbonate. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. It turns from. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
UK GCSE Chemistry Topic 1 Zinc Carbonate YouTube Zinc Carbonate Decomposition Reaction Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. The aim of this experiment is to compare the reactivity of some different metal carbonates. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Copper, zinc and calcium carbonate. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate Decomposition Reaction The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. Metal carbonates decompose when heated. The compound named zinc carbonate undergoes decomposition in the presence of heat to form. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. Compare. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate Decomposition Reaction Cuco3 (s) → cuo (s) + co2 (g) examiner tip. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. Metal carbonates decompose when heated. The. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
Type of Reaction for ZnCO3 = ZnO + CO2 YouTube Zinc Carbonate Decomposition Reaction The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. The aim of this experiment is to compare the reactivity of some different metal carbonates. Metal carbonates decompose when heated. The compound named zinc carbonate undergoes decomposition in the presence of heat to form. Copper carbonate decomposes at 290 °c. The reaction of. Zinc Carbonate Decomposition Reaction.
From www.expii.com
Reactions — Definition & Examples Expii Zinc Carbonate Decomposition Reaction It turns from green to black when heated. Metal carbonates decompose when heated. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Copper, zinc and calcium carbonate will decompose. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
Metal Carbonate YouTube Zinc Carbonate Decomposition Reaction Metal carbonates decompose when heated. It turns from green to black when heated. Copper carbonate decomposes at 290 °c. The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. Synthesis. Zinc Carbonate Decomposition Reaction.
From www.numerade.com
SOLVED The of zinc carbonate, \mathrm{ZnCO}_{3}(\mathrm Zinc Carbonate Decomposition Reaction The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. Some carbonates are more reactive than others. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
reaction of lead nitrate class 10 of lead Zinc Carbonate Decomposition Reaction Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Metal carbonates decompose when heated. Make sure you know the observations for the decomposition for copper (ii) carbonate. Reaction of zinc carbonate upon heating: The compound named zinc carbonate undergoes decomposition in the presence of heat. Zinc Carbonate Decomposition Reaction.
From www.numerade.com
Lead (II) carbonate to form lead (II) oxide and carbon Zinc Carbonate Decomposition Reaction Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Some carbonates are more reactive than others. The aim of this experiment is to compare the reactivity of some different metal carbonates. Reaction of zinc carbonate upon heating: Cuco3 (s) → cuo (s) + co2 (g) examiner tip. The thermal. Zinc Carbonate Decomposition Reaction.
From www.researchgate.net
(PDF) The of Zinc Carbonate Using Stoichiometry To Zinc Carbonate Decomposition Reaction The compound named zinc carbonate undergoes decomposition in the presence of heat to form. Reaction of zinc carbonate upon heating: Cuco3 (s) → cuo (s) + co2 (g) examiner tip. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and. Zinc Carbonate Decomposition Reaction.
From www.toppr.com
Identify the type of reactions taking place in each of the following Zinc Carbonate Decomposition Reaction Metal carbonates decompose when heated. Make sure you know the observations for the decomposition for copper (ii) carbonate. Copper carbonate decomposes at 290 °c. The compound named zinc carbonate undergoes decomposition in the presence of heat to form. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
of Copper Carbonate YouTube Zinc Carbonate Decomposition Reaction The compound named zinc carbonate undergoes decomposition in the presence of heat to form. Reaction of zinc carbonate upon heating: Copper carbonate decomposes at 290 °c. Make sure you know the observations for the decomposition for copper (ii) carbonate. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Copper,. Zinc Carbonate Decomposition Reaction.
From www.numerade.com
The of zinc carbonate, ZnCO3( s), into zinc oxide, ZnO(s Zinc Carbonate Decomposition Reaction Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. The compound named zinc. Zinc Carbonate Decomposition Reaction.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Carbonate Decomposition Reaction The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. Some carbonates are more reactive than others. The aim of this experiment is to compare the reactivity of some different metal carbonates. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will. Zinc Carbonate Decomposition Reaction.
From www.slideserve.com
PPT What is a chemical reaction? PowerPoint Presentation, free Zinc Carbonate Decomposition Reaction The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. Cuco3 (s) → cuo (s) + co2 (g) examiner tip. The compound named zinc carbonate undergoes decomposition in the presence. Zinc Carbonate Decomposition Reaction.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the Zinc Carbonate Decomposition Reaction Make sure you know the observations for the decomposition for copper (ii) carbonate. It turns from green to black when heated. Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. The compound named zinc carbonate undergoes decomposition in the presence of heat to form. Compare the thermal stabilities of carbonates of reactive metals,. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Carbonate Decomposition Reaction Make sure you know the observations for the decomposition for copper (ii) carbonate. It turns from green to black when heated. The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. Reaction of zinc carbonate upon heating: Results showed that zinc carbonate hydroxide decomposition started at about. Zinc Carbonate Decomposition Reaction.
From www.nagwa.com
Question Video Finding the Mass of the Products in a Thermal Zinc Carbonate Decomposition Reaction Make sure you know the observations for the decomposition for copper (ii) carbonate. Cuco3 (s) → cuo (s) + co2 (g) examiner tip. The compound named zinc carbonate undergoes decomposition in the presence of heat to form. The aim of this experiment is to compare the reactivity of some different metal carbonates. The thermal decomposition of zinc carbonate has been. Zinc Carbonate Decomposition Reaction.
From sciencenotes.org
What Is a Reaction? Definition and Examples Zinc Carbonate Decomposition Reaction Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Cuco3 (s) → cuo (s) + co2 (g) examiner tip. Make sure you know the observations for the decomposition for copper (ii) carbonate. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. Reaction. Zinc Carbonate Decomposition Reaction.
From www.slideserve.com
PPT CALCIUM CARBONATE A guide for GCSE students PowerPoint Zinc Carbonate Decomposition Reaction Copper, zinc and calcium carbonate will decompose at the temperature that a bunsen burner will reach. Cuco3 (s) → cuo (s) + co2 (g) examiner tip. Some carbonates are more reactive than others. The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. The compound named zinc carbonate undergoes decomposition in the presence. Zinc Carbonate Decomposition Reaction.
From diya-jolpblogcooper.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid Zinc Carbonate Decomposition Reaction The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. The aim of this experiment is to compare the reactivity of some different metal carbonates. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained via chemical. Results showed. Zinc Carbonate Decomposition Reaction.
From www.youtube.com
Synthesis of basic Zinc Carbonate YouTube Zinc Carbonate Decomposition Reaction Cuco3 (s) → cuo (s) + co2 (g) examiner tip. The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures between 200 and 260°c. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Synthesis of zinc oxide is often achieved by thermal. Zinc Carbonate Decomposition Reaction.
From www.slideserve.com
PPT Reactions PowerPoint Presentation, free download Zinc Carbonate Decomposition Reaction Some carbonates are more reactive than others. Metal carbonates decompose when heated. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. Copper carbonate decomposes at 290 °c. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead. Zinc Carbonate Decomposition Reaction.
From www.freepik.com
Premium Vector of calcium carbonate vector illustration Zinc Carbonate Decomposition Reaction The reaction of basic zinc carbonate (2znco3.3zn(oh)2.h20) is believed to produce both co2 and h20 at the same interface and to form no. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Make sure you know the observations for the decomposition for copper (ii) carbonate.. Zinc Carbonate Decomposition Reaction.
From www.shutterstock.com
Reaction Infographic Diagram Example Zinc Stock Vector Zinc Carbonate Decomposition Reaction Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Make sure you know the observations for the decomposition for copper (ii) carbonate. The compound named zinc carbonate undergoes decomposition in the presence of heat to form. Cuco3 (s) → cuo (s) + co2 (g) examiner. Zinc Carbonate Decomposition Reaction.
From www.slideserve.com
PPT Thermal PowerPoint Presentation, free download ID Zinc Carbonate Decomposition Reaction Copper carbonate decomposes at 290 °c. Reaction of zinc carbonate upon heating: The aim of this experiment is to compare the reactivity of some different metal carbonates. Results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition became significant at. The thermal decomposition of zinc carbonate has been studied isothermally at various temperatures. Zinc Carbonate Decomposition Reaction.
From www.vectorstock.com
reaction copper carbonate Vector Image Zinc Carbonate Decomposition Reaction Cuco3 (s) → cuo (s) + co2 (g) examiner tip. It turns from green to black when heated. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Synthesis of zinc oxide is often achieved by thermal decomposition of the precursor of basic zinc carbonate obtained. Zinc Carbonate Decomposition Reaction.