Aluminum Oxide Positive Ion . two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative. Next, write the base of the name of. remember that ions are formed only when electrons move from one atom to another; Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. to write the name of this compound name the positive ion (cation) first. Aluminium oxide reacts with hot dilute. A proton never moves from one atom to.
from www.wou.edu
remember that ions are formed only when electrons move from one atom to another; Aluminium oxide reacts with hot dilute. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. to write the name of this compound name the positive ion (cation) first. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Next, write the base of the name of. A proton never moves from one atom to.
CH150 Chapter 5 Chemical Reactions Chemistry
Aluminum Oxide Positive Ion the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Next, write the base of the name of. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute. remember that ions are formed only when electrons move from one atom to another; to write the name of this compound name the positive ion (cation) first. A proton never moves from one atom to.
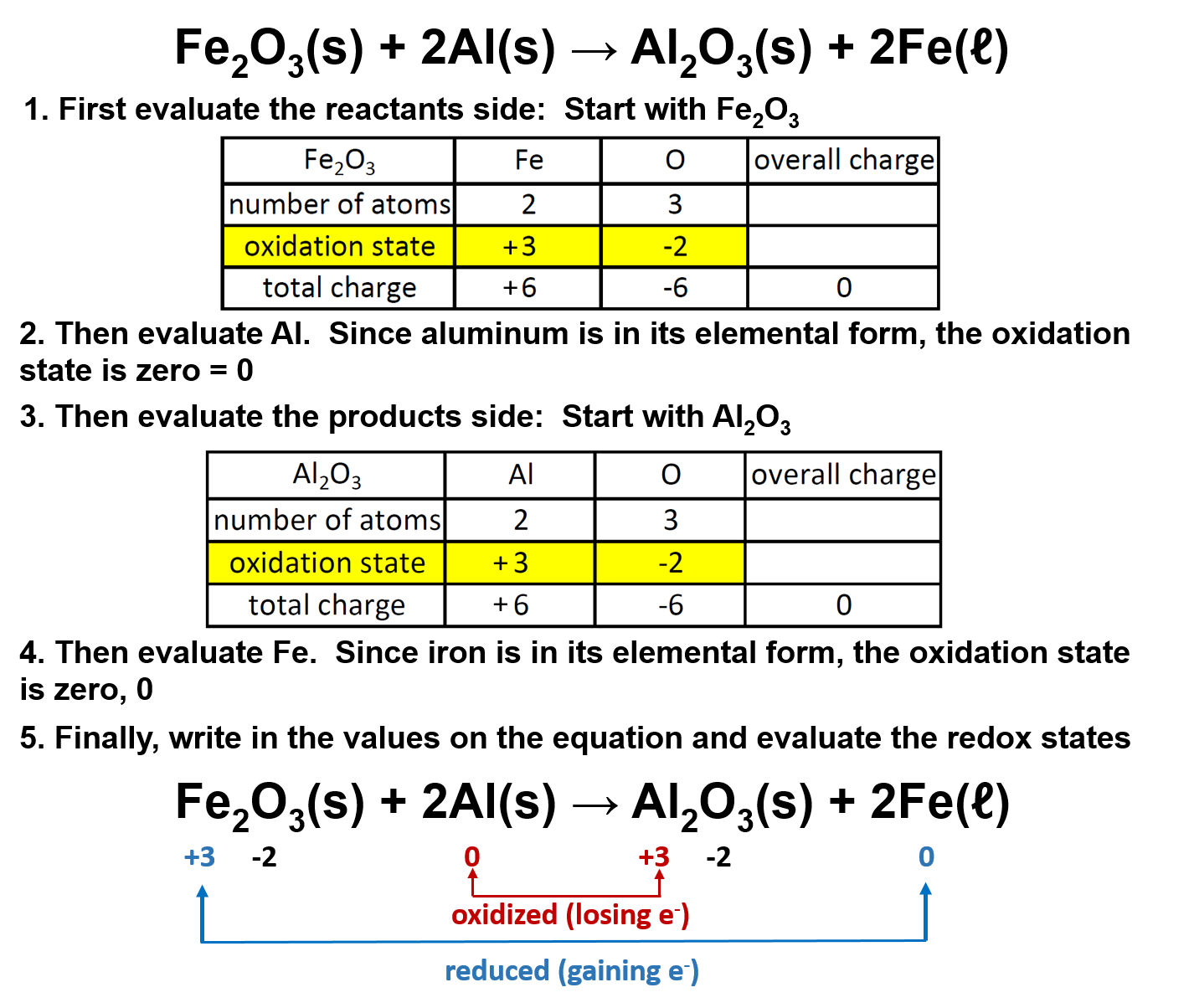
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Aluminum Oxide Positive Ion A proton never moves from one atom to. Next, write the base of the name of. Aluminium oxide reacts with hot dilute. remember that ions are formed only when electrons move from one atom to another; the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. . Aluminum Oxide Positive Ion.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Aluminum Oxide Positive Ion Next, write the base of the name of. remember that ions are formed only when electrons move from one atom to another; to write the name of this compound name the positive ion (cation) first. A proton never moves from one atom to. aluminium oxide contains oxide ions, and thus reacts with acids in the same way. Aluminum Oxide Positive Ion.
From study.com
Aluminum Oxide Compound Formula, Properties & Structure Video Aluminum Oxide Positive Ion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of.. Aluminum Oxide Positive Ion.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Aluminum Oxide Positive Ion to write the name of this compound name the positive ion (cation) first. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge. Aluminum Oxide Positive Ion.
From www.youtube.com
Is Al2O3 (Aluminum oxide) Ionic or Covalent? YouTube Aluminum Oxide Positive Ion the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Next, write the base of the name of. to write the name of this compound name the. Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID4539778 Aluminum Oxide Positive Ion Aluminium oxide reacts with hot dilute. A proton never moves from one atom to. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Next, write the base of the. Aluminum Oxide Positive Ion.
From www.science-revision.co.uk
Extracting aluminium Aluminum Oxide Positive Ion Next, write the base of the name of. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative. . Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT Do now! PowerPoint Presentation ID2938610 Aluminum Oxide Positive Ion the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Aluminium oxide reacts with hot dilute. A proton never moves from one atom to. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. to write the name of. Aluminum Oxide Positive Ion.
From www.youtube.com
Give formation of Aluminium oxide Formation of aluminium oxide by Aluminum Oxide Positive Ion aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative. A proton never. Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT Chemical Nomenclature PowerPoint Presentation, free download ID Aluminum Oxide Positive Ion the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. to write the name of this compound name the positive ion (cation) first. Aluminium oxide reacts with hot dilute. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three. Aluminum Oxide Positive Ion.
From www.science-revision.co.uk
Extracting aluminium Aluminum Oxide Positive Ion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Next, write the base of the name of. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. the formula of an ionic compound represents the simplest ratio of the numbers. Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Aluminum Oxide Positive Ion to write the name of this compound name the positive ion (cation) first. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−,. Aluminum Oxide Positive Ion.
From falibert.blogspot.com
Electrolysis Of Aluminium Oxide Extraction of Aluminium by Aluminum Oxide Positive Ion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions,. Aluminum Oxide Positive Ion.
From www.youtube.com
Lewis Dot Structure for Al2O3 (Aluminum Oxide) YouTube Aluminum Oxide Positive Ion Next, write the base of the name of. A proton never moves from one atom to. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. remember that ions are formed only when electrons move from one atom to another; to write the name of this compound name. Aluminum Oxide Positive Ion.
From www.goodscience.com.au
Determining the Formula for Ionic Compounds Good Science Aluminum Oxide Positive Ion Next, write the base of the name of. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative. . Aluminum Oxide Positive Ion.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma Aluminum Oxide Positive Ion remember that ions are formed only when electrons move from one atom to another; Aluminium oxide reacts with hot dilute. Next, write the base of the name of. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Al is a type i monatomic ion, no roman numeral is. Aluminum Oxide Positive Ion.
From www.youtube.com
Writing the Formula for Aluminum Oxide YouTube Aluminum Oxide Positive Ion aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of.. Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID5648526 Aluminum Oxide Positive Ion the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. A proton never moves from one atom to. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six. Aluminum Oxide Positive Ion.
From www.researchgate.net
Schematic twodimensional representation of aluminum oxide structures Aluminum Oxide Positive Ion remember that ions are formed only when electrons move from one atom to another; two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative. aluminium oxide contains oxide ions, and thus reacts with acids in the same. Aluminum Oxide Positive Ion.
From www.youtube.com
Aluminium Oxide Properties, Productions and Applications YouTube Aluminum Oxide Positive Ion Aluminium oxide reacts with hot dilute. A proton never moves from one atom to. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Next, write the base. Aluminum Oxide Positive Ion.
From ar.inspiredpencil.com
Aluminum Oxide Lewis Structure Aluminum Oxide Positive Ion Aluminium oxide reacts with hot dilute. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. remember that ions are formed only when electrons move from one atom to another; two aluminum ions, each with a charge of 3+, would give us six positive charges, and. Aluminum Oxide Positive Ion.
From chemistry291.blogspot.com
What Is the Chemical Formula for Aluminum Oxide? Aluminum Oxide Positive Ion the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. A proton never moves from one atom to. remember that ions are formed only when electrons move from one atom to another; Al is a type i monatomic ion, no roman numeral is needed since the charge. Aluminum Oxide Positive Ion.
From chemistry291.blogspot.com
Formula for Aluminum OxideWhat is the Chemical Formula for Aluminum Aluminum Oxide Positive Ion aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. remember that ions are formed only when electrons move from one atom to another; two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of. Aluminum Oxide Positive Ion.
From stonecoldhands.com
Ionic Compounds Aluminum Oxide Positive Ion Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. Aluminium oxide reacts with hot dilute. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative. remember that ions are. Aluminum Oxide Positive Ion.
From ubicaciondepersonas.cdmx.gob.mx
Aluminum Oxide Structure ubicaciondepersonas.cdmx.gob.mx Aluminum Oxide Positive Ion remember that ions are formed only when electrons move from one atom to another; Aluminium oxide reacts with hot dilute. to write the name of this compound name the positive ion (cation) first. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of. Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT WarmUp PowerPoint Presentation, free download ID3731178 Aluminum Oxide Positive Ion A proton never moves from one atom to. aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute. to write the name of this compound name the positive ion (cation) first. remember that ions are formed only when electrons move from one. Aluminum Oxide Positive Ion.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.37 Understand How Ions are formed by Electron Aluminum Oxide Positive Ion remember that ions are formed only when electrons move from one atom to another; aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. A proton never moves from one atom to. to write the name of this compound name the positive ion (cation) first. the formula. Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint Aluminum Oxide Positive Ion to write the name of this compound name the positive ion (cation) first. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge. Aluminum Oxide Positive Ion.
From www.youtube.com
Al 3+ Electron Configuration (Aluminum Ion) YouTube Aluminum Oxide Positive Ion remember that ions are formed only when electrons move from one atom to another; Next, write the base of the name of. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. A proton never moves from one atom to. to write the name of this compound name the positive. Aluminum Oxide Positive Ion.
From ar.inspiredpencil.com
Aluminum Oxide Structure Aluminum Oxide Positive Ion two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative. Aluminium oxide reacts with hot dilute. to write the name of this compound name the positive ion (cation) first. Next, write the base of the name of. . Aluminum Oxide Positive Ion.
From www.youtube.com
How to write the equation for Al2O3 + H2O (Aluminum oxide + Water Aluminum Oxide Positive Ion remember that ions are formed only when electrons move from one atom to another; aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Next, write the. Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Aluminum Oxide Positive Ion aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Next, write the base of the name of. A proton never moves. Aluminum Oxide Positive Ion.
From www.pw.live
Aluminium Oxide Formula, Structure, Properties, Uses Aluminum Oxide Positive Ion the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Aluminium oxide reacts with hot dilute. Next, write the base of the name of. to write the name of this compound name the positive ion (cation) first. remember that ions are formed only when electrons move. Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Aluminum Oxide Positive Ion to write the name of this compound name the positive ion (cation) first. A proton never moves from one atom to. remember that ions are formed only when electrons move from one atom to another; aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. the formula. Aluminum Oxide Positive Ion.
From www.slideserve.com
PPT Chapter 19— CHEMICAL BONDS PowerPoint Presentation, free download Aluminum Oxide Positive Ion the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Next, write the base of the name of. Al is a type i monatomic ion, no roman numeral is needed since the charge does not change. two aluminum ions, each with a charge of 3+, would give. Aluminum Oxide Positive Ion.