Iron (Iii) Chloride Observation . characteristic reactions of fe²⁺ and fe³⁺. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Select the option from the observation column that best matches. The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. Observations when a few drops of sodium. Salicylic acid result use the observations you have made to complete the following table. note your observations in the following table. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. Or you can test any. In aqueous solutions, an electrical double layer (edl). Name of formula and ion.
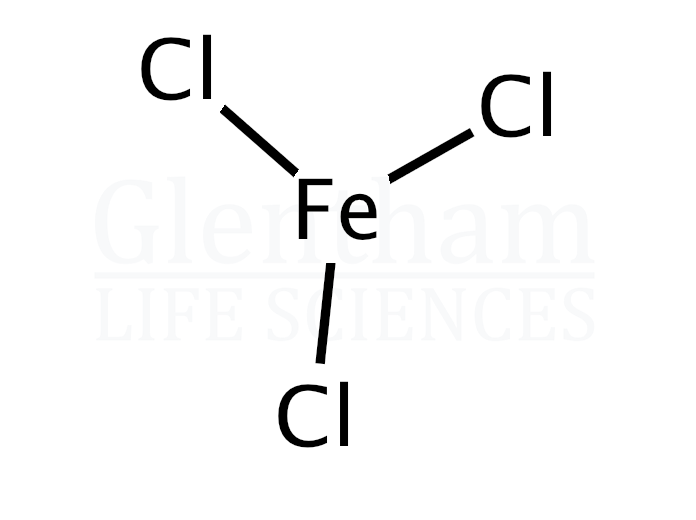
from www.glentham.com
characteristic reactions of fe²⁺ and fe³⁺. Salicylic acid result use the observations you have made to complete the following table. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Observations when a few drops of sodium. Name of formula and ion. Select the option from the observation column that best matches. Or you can test any. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake.
Iron(III) chloride, anhydrous (CAS 7705080) Glentham Life Sciences
Iron (Iii) Chloride Observation Or you can test any. Observations when a few drops of sodium. Salicylic acid result use the observations you have made to complete the following table. Name of formula and ion. Select the option from the observation column that best matches. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. Or you can test any. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. note your observations in the following table. In aqueous solutions, an electrical double layer (edl). The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. characteristic reactions of fe²⁺ and fe³⁺.
From hdhg2022.en.made-in-china.com
Iron (III) Chloride China Iron (III) Chloride and Fecl3 Iron (Iii) Chloride Observation you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Observations when a few drops of sodium. Salicylic acid result use the observations you have made to complete the following table. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the. Iron (Iii) Chloride Observation.
From shop.porphychem.com
iron(III) tetraphenylporphyrin chloride CAS 116456818 PorphyChem Iron (Iii) Chloride Observation Select the option from the observation column that best matches. Salicylic acid result use the observations you have made to complete the following table. Or you can test any. Observations when a few drops of sodium. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. The [fe(h2o)6]3+ [fe (h 2. Iron (Iii) Chloride Observation.
From www.anugrahputrakencana.co.id
Jual Iron III Chloride Hexahydrate oleh PT. ANUGRAH PUTRA KENCANA Iron (Iii) Chloride Observation you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Select the option from the observation column that best matches. Salicylic acid result use the observations you have made to complete the following table. Or you can test any. characteristic reactions of fe²⁺ and fe³⁺. In aqueous solutions,. Iron (Iii) Chloride Observation.
From www.docdroid.net
Iron(III) chloride anhydrous SA.pdf DocDroid Iron (Iii) Chloride Observation you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Salicylic acid result use the observations you have made to complete the following table. Select the option from the observation column that best matches. Observations when a few drops of sodium. characteristic reactions of fe²⁺ and fe³⁺. . Iron (Iii) Chloride Observation.
From www.youtube.com
Does Iron (III) Chloride/FeCl3 react with aspirin or salicylic acid Iron (Iii) Chloride Observation investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. Select the option from the observation column that best matches. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. Name of formula and ion. Observations when a few drops. Iron (Iii) Chloride Observation.
From www.scientificlabs.ie
Iron(III) chloride, , sublimed 7011225G SIGMA ALDRICH SLS Ireland Iron (Iii) Chloride Observation Name of formula and ion. note your observations in the following table. Select the option from the observation column that best matches. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. Or you can test any. iron(iii) ions and thiocyanate ions react in solution to produce. Iron (Iii) Chloride Observation.
From www.mbizmarket.co.id
IRON (III) CHLORIDE 250 GR 1.03943.0250 Iron (Iii) Chloride Observation Observations when a few drops of sodium. In aqueous solutions, an electrical double layer (edl). The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. Or you can test any. Name of formula and ion. you can use a few milliliters of either the. Iron (Iii) Chloride Observation.
From www.chegg.com
Solved A 5.49 g sample of an iron (III) chloride hydrate was Iron (Iii) Chloride Observation Salicylic acid result use the observations you have made to complete the following table. Or you can test any. In aqueous solutions, an electrical double layer (edl). you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. iron(iii) ions and thiocyanate ions react in solution to produce the. Iron (Iii) Chloride Observation.
From vittascientific.com
Iron (III) Chloride Anhydrous (Ferric) 250g(BN/EXP) VITTA Scientific Iron (Iii) Chloride Observation Salicylic acid result use the observations you have made to complete the following table. note your observations in the following table. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Observations when a few drops of sodium. characteristic reactions of fe²⁺ and fe³⁺. Name of formula. Iron (Iii) Chloride Observation.
From hdhg2022.en.made-in-china.com
Ferric Chloride China Iron (III) Chloride and Fecl3 Iron (Iii) Chloride Observation Observations when a few drops of sodium. In aqueous solutions, an electrical double layer (edl). investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. note your observations in the following table. The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but. Iron (Iii) Chloride Observation.
From melscience.com
The hydrolysis of iron(III) chloride MEL Chemistry Iron (Iii) Chloride Observation iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. characteristic reactions of fe²⁺ and fe³⁺. Observations when a few drops of sodium. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. In aqueous solutions, an electrical double. Iron (Iii) Chloride Observation.
From www.youtube.com
How to Write the Formula for Iron (III) chloride YouTube Iron (Iii) Chloride Observation The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. characteristic reactions of fe²⁺ and fe³⁺. Salicylic acid result use the observations you have made to complete the following table. Select the option from the observation column that best matches. note your observations. Iron (Iii) Chloride Observation.
From www.researchgate.net
Raman spectra of iron(III) chloride in the solid, liquid and gaseous Iron (Iii) Chloride Observation Name of formula and ion. note your observations in the following table. Select the option from the observation column that best matches. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. Observations when a few drops of sodium. Salicylic acid result use the observations you have made. Iron (Iii) Chloride Observation.
From slideplayer.com
Chapter 7 Compounds and Their Bonds ppt download Iron (Iii) Chloride Observation Name of formula and ion. The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. characteristic reactions of fe²⁺ and fe³⁺. Salicylic acid result use the observations you have made to complete the following table. In aqueous solutions, an electrical double layer (edl). Observations. Iron (Iii) Chloride Observation.
From www.youtube.com
Write the formula for iron(III) chloride YouTube Iron (Iii) Chloride Observation Salicylic acid result use the observations you have made to complete the following table. note your observations in the following table. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. Name of formula and ion. Or you can test any. characteristic reactions of fe²⁺ and fe³⁺. In aqueous. Iron (Iii) Chloride Observation.
From www.chegg.com
Solved Thiocyanatoiron(III) Ion Data and Observations In Iron (Iii) Chloride Observation Select the option from the observation column that best matches. note your observations in the following table. Salicylic acid result use the observations you have made to complete the following table. The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. In aqueous solutions,. Iron (Iii) Chloride Observation.
From www.chegg.com
Solved Iron(III) chloride + Potassium phosphate Yellowbrown Iron (Iii) Chloride Observation The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. Name of formula and ion. characteristic reactions of fe²⁺ and fe³⁺. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. investigate. Iron (Iii) Chloride Observation.
From chemistryexperimentphotogallery.blogspot.com
Chemistry Experiment Photo Gallery chapter 8 Form 4 Salts Iron (Iii) Chloride Observation characteristic reactions of fe²⁺ and fe³⁺. note your observations in the following table. The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. Or you can test any. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii). Iron (Iii) Chloride Observation.
From www.sciencephoto.com
Iron (III) chloride hexahydrate Stock Image C039/0846 Science Iron (Iii) Chloride Observation Salicylic acid result use the observations you have made to complete the following table. In aqueous solutions, an electrical double layer (edl). Observations when a few drops of sodium. The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. Select the option from the observation. Iron (Iii) Chloride Observation.
From www.sciencephoto.com
Sodium hydroxide and iron III chloride Stock Image C028/4185 Iron (Iii) Chloride Observation iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. In aqueous solutions, an electrical double layer (edl). Select the option from the observation column that best matches. characteristic reactions of fe²⁺ and fe³⁺. Salicylic acid result use the observations you have made to complete the following table. you. Iron (Iii) Chloride Observation.
From www.isolab.de
ISOLAB IRON(III) CHLORIDE HEXAHYDRATE CAS No 10025771 ISOLAB Iron (Iii) Chloride Observation Name of formula and ion. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. note your observations in the following table. Salicylic acid result use the observations you have made to complete the following table. you can use a few milliliters of either the iron(ii) sulfate. Iron (Iii) Chloride Observation.
From icsechemistry16.blogspot.com
Preparation of Iron (III) chloride (Ferric chloride) Iron (Iii) Chloride Observation In aqueous solutions, an electrical double layer (edl). iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. note your observations in the following table. The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber.. Iron (Iii) Chloride Observation.
From hoachatthinghiem.org
Iron (III) Chloride Hexahydrate (FeCl3.6H2O, AR, Chai 500G, Xilong, Cas Iron (Iii) Chloride Observation The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. In aqueous solutions, an electrical double layer (edl). you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Or you can test any. . Iron (Iii) Chloride Observation.
From fphoto.photoshelter.com
science chemistry compound iron iii chloride Fundamental Photographs Iron (Iii) Chloride Observation Salicylic acid result use the observations you have made to complete the following table. Name of formula and ion. The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. In aqueous solutions, an electrical double layer (edl). investigate the aqueous reaction of potassium thiocyanate. Iron (Iii) Chloride Observation.
From www.chegg.com
Solved Name Part B. Iron(III) Chloride (FeCl) plus Potassium Iron (Iii) Chloride Observation Or you can test any. Name of formula and ion. note your observations in the following table. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Salicylic acid result use the observations you have made to complete the following table. Select the option from the observation column. Iron (Iii) Chloride Observation.
From www.fishersci.se
Iron(III) chloride, 98, pure, anhydrous, Thermo Scientific Chemicals Iron (Iii) Chloride Observation Select the option from the observation column that best matches. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. characteristic reactions of fe²⁺ and fe³⁺. you can. Iron (Iii) Chloride Observation.
From shopee.co.th
Iron(III) Chloride Hexahydrate Grade AR 1KG (QRec) Shopee Thailand Iron (Iii) Chloride Observation investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. Name of formula and ion. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Observations when a few drops of sodium. Select the option from the observation. Iron (Iii) Chloride Observation.
From herbitas.com
Iron III Chloride 40 Iron (Iii) Chloride Observation The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. you can use a few milliliters of either the iron(ii) sulfate solution or. Iron (Iii) Chloride Observation.
From hdhg2022.en.made-in-china.com
Iron Chloride Anhydrous China Iron (III) Chloride and Fecl3 Iron (Iii) Chloride Observation Or you can test any. Name of formula and ion. In aqueous solutions, an electrical double layer (edl). characteristic reactions of fe²⁺ and fe³⁺. Salicylic acid result use the observations you have made to complete the following table. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown.. Iron (Iii) Chloride Observation.
From www.slideserve.com
PPT Binary Molecular PowerPoint Presentation, free download ID4566789 Iron (Iii) Chloride Observation The [fe(h2o)6]3+ [fe (h 2 o) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. note your observations in the following table. Salicylic acid result use the observations. Iron (Iii) Chloride Observation.
From fineartamerica.com
Iron Chlorides Photograph by Martyn F. Chillmaid/science Photo Library Iron (Iii) Chloride Observation Salicylic acid result use the observations you have made to complete the following table. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. Observations when a few drops of sodium. characteristic reactions of fe²⁺ and fe³⁺. Select the option from the observation column that best matches. note your. Iron (Iii) Chloride Observation.
From www.fishersci.co.uk
Iron(III) chloride hexahydrate, 99+, for analysis, Thermo Scientific Iron (Iii) Chloride Observation Select the option from the observation column that best matches. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Name of formula and ion. Salicylic acid result. Iron (Iii) Chloride Observation.
From chemistry-chemists.com
Iron chloride (III) and boiling water. Хлорид железа (III) и кипящая Iron (Iii) Chloride Observation Salicylic acid result use the observations you have made to complete the following table. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Observations when a few drops of sodium. In aqueous solutions, an electrical double layer (edl). investigate the aqueous reaction of potassium thiocyanate with iron(iii). Iron (Iii) Chloride Observation.
From www.glentham.com
Iron(III) chloride, anhydrous (CAS 7705080) Glentham Life Sciences Iron (Iii) Chloride Observation characteristic reactions of fe²⁺ and fe³⁺. note your observations in the following table. Name of formula and ion. investigate the aqueous reaction of potassium thiocyanate with iron(iii) nitrate that they have heard other companies are using as fake. Salicylic acid result use the observations you have made to complete the following table. iron(iii) ions and thiocyanate. Iron (Iii) Chloride Observation.
From www.sciencephoto.com
Iron(III) chloride hexahydrate Stock Image C027/9269 Science Iron (Iii) Chloride Observation Observations when a few drops of sodium. iron(iii) ions and thiocyanate ions react in solution to produce the complex ion thiocyanotoiron(iii) according to the equation. you can use a few milliliters of either the iron(ii) sulfate solution or the iron(iii) chloride solution as the unknown. Salicylic acid result use the observations you have made to complete the following. Iron (Iii) Chloride Observation.